Supplemental Insulin-Like Growth Factor-1 and Necrotizing Enterocolitis in Preterm Pigs
- PMID: 33614541
- PMCID: PMC7891102
- DOI: 10.3389/fped.2020.602047
Supplemental Insulin-Like Growth Factor-1 and Necrotizing Enterocolitis in Preterm Pigs
Abstract
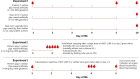
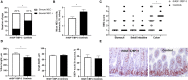
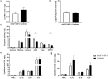
Background: Recombinant human IGF-1/binding protein-3 (rhIGF-1/BP-3) is currently tested as a therapy in preterm infants but possible effects on the gut, including necrotizing enterocolitis (NEC), have not been tested. The aim of this study was to evaluate if rhIGF-1/BP-3 supplementation in the first days after birth negatively affects clinical variables like growth, physical activity, blood chemistry and hematology and gut maturation (e.g., intestinal permeability, morphology, enzyme activities, cytokine levels, enterocyte proliferation, NEC lesions), using NEC-sensitive preterm pigs as a model for preterm infants. Methods: Preterm pigs were given twice daily subcutaneous injections of rhIGF-1/BP-3 or vehicle. Blood was collected for IGF-1 measurements and gut tissue for NEC evaluation and biochemical analyses on day 5. Results: Baseline circulating IGF-1 levels were low in preterm pigs compared with near-term pigs reared by their mother (<20 vs. 70 ng/ml). Injection with rhIGF-1/BP-3 resulted in increased plasma IGF-1 levels for up to 6 h after injection (>40 ng/mL). rhIGF-1/BP-3 treatment reduced the incidence of severe NEC lesions (7/24 vs.16/24, p = 0.01) and overall NEC severity (1.8 ± 0.2 vs. 2.6 ± 0.3, p < 0.05, with most lesions occurring in colon). In the small intestine, villi length (405 ± 25 vs. 345 ± 33 μm) and activities of the brush border peptidases aminopeptidase N and dipeptidylpeptidase IV were increased in rhIGF-1/BP-3 treated pigs, relative to control pigs (+31-44%, both p < 0.05). The treatment had no effects on body weight, blood chemistry or hematology, except for an increase in blood leucocyte and neutrophil counts (p < 0.05, i.e., reduced neonatal neutropenia). Likewise, rhIGF-1/BP-3 treatment did not affect intestinal tissue cytokine levels (IL-1β, IL-6, IL-8, TNFα,), enterocyte proliferation, goblet cell density, permeability or bacterial translocation to the bone marrow. Conclusion: Supplemental rhIGF-1/BP-3 did not negatively affect any of the measured variables of clinical status or gut maturation in preterm pigs. Longer-term safety and efficacy of exogenous rhIGF-1/BP-3 to support maturation of the gut and other critical organs in preterm newborns remain to be investigated in both pigs and infants.
Keywords: IGF-1; fetus; growth restriction; gut; infant; intestine; newborn; preterm birth.
Copyright © 2021 Holgersen, Gao, Narayanan, Gaur, Carey, Barton, Pan, Muk, Thymann and Sangild.
Conflict of interest statement
TG, NB, RN, and GC were employed at Takeda, MA at the time of study. These co-authors did not participate in study execution, data acquisition or in drafting the manuscript with its text, results presentation, tables and figures. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. (2012) 379:2162–72. 10.1016/S0140-6736(12)60820-4 - DOI - PubMed
-
- Diaz-Gomez NM, Domenech E, Barroso F. Breast-feeding and growth factors in preterm newborn infants. J pediatr gastroenterol nutr. (1997) 24:322–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

