High-Level Patchoulol Biosynthesis in Artemisia annua L
- PMID: 33614607
- PMCID: PMC7890116
- DOI: 10.3389/fbioe.2020.621127
High-Level Patchoulol Biosynthesis in Artemisia annua L
Abstract
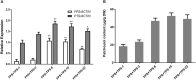
Terpenes constitute the largest class of secondary metabolites in plants. Some terpenes are essential for plant growth and development, membrane components, and photosynthesis. Terpenes are also economically useful for industry, agriculture, and pharmaceuticals. However, there is very low content of most terpenes in microbes and plants. Chemical or microbial synthesis of terpenes are often costly. Plants have the elaborate and economic biosynthetic way of producing high-value terpenes through photosynthesis. Here we engineered the heterogenous sesquiterpenoid patchoulol production in A. annua. When using a strong promoter such as 35S to over express the avian farnesyl diphosphate synthase gene and patchoulol synthase gene, the highest content of patchoulol was 52.58 μg/g DW in transgenic plants. When altering the subcellular location of the introduced sesquiterpene synthetase via a signal peptide, the accumulation of patchoulol was observably increased to 273 μg/g DW. This case demonstrates that A. annua plant with glandular trichomes is a useful platform for synthetic biology studies.
Keywords: Artemisia annua L.; patchoulol; sesquiterpenoids; synthetic biology; terpenes.
Copyright © 2021 Fu, Zhang, Ma, Hassani, Peng, Pan, Zhang, Deng, Liu, Zhang, Han, Chen, Zhao, Li, Sun and Tang.
Conflict of interest statement
YZ, ZD, WL, JZhang, LH, and DC were employed by company Firmenich Aromatics (China) Co. Ltd. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bertea C. M., Voster A., Verstappen F. W., Maffei M., Beekwilder J., Bouwmeester H. J. (2006). Isoprenoid biosynthesis in Artemisia annua: cloning and heterologous expression of a germacrene A synthase from a glandular trichome cDNA library. Arch. Biochem. Biophys. 448, 3–12. 10.1016/j.abb.2006.02.026 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

