The BMP2 Signaling Axis Promotes Invasive Differentiation of Human Trophoblasts
- PMID: 33614644
- PMCID: PMC7889606
- DOI: 10.3389/fcell.2021.607332
The BMP2 Signaling Axis Promotes Invasive Differentiation of Human Trophoblasts
Abstract
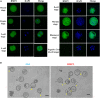
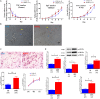
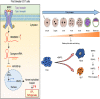
Embryo implantation and trophoblast invasion are principal limiting factors of pregnancy establishment. Aberrant embryo development or improper trophoblast differentiation and invasion may lead to various unfavorable pregnancy-related outcomes, including early pregnancy loss (EPL). Our clinical data show that the serum BMP2 levels were significantly increased during the first trimester of pregnancy and that the serum and BMP2 expression levels were lower in women with EPL than in women with normal early pregnancies. Moreover, we observed that BMP2 was expressed in oocytes and trophoblast cells of cleaved embryos and blastocysts prior to implantation in both humans and mice. Exogenous BMP2 promoted embryonic development by enhancing blastocyst formation and hatching in mice. LncRNA NR026833.1 was upregulated by BMP2 and promoted SNAIL expression by competitively binding to miR-502-5p. SNAIL induced MMP2 expression and promoted cell invasion in primary extravillous trophoblast cells. BMP2 promotes the invasive differentiation of mouse trophoblast stem cells by downregulating the expression of TS cell marker and upregulating the expression of trophoblast giant cell marker and labyrinthine/spongiotrophoblast marker. Our findings provide significant insights into the regulatory roles of BMP2 in the development of the placenta, which may give us a framework to explore new therapeutic strategies to pregnancy-related complications.
Keywords: BMP2; early pregnancy loss; embryo development; lncRNA; trophoblast differentiation; trophoblast invasion.
Copyright © 2021 You, Wang, Chang, Yi, Zhao, Zhu, Sun, Tang, Wang, Sang, Feng, Cheng, Leung and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Bone morphogenetic protein 2 promotes human trophoblast cell invasion and endothelial-like tube formation through ID1-mediated upregulation of IGF binding protein-3.FASEB J. 2020 Feb;34(2):3151-3164. doi: 10.1096/fj.201902168RR. Epub 2020 Jan 3. FASEB J. 2020. PMID: 31908038
-
Transcription factor SOX4 facilitates BMP2-regulated gene expression during invasive trophoblast differentiation.FASEB J. 2021 Dec;35(12):e22028. doi: 10.1096/fj.202100925RR. FASEB J. 2021. PMID: 34739154
-
Bone morphogenetic protein 2 promotes human trophoblast cell invasion by upregulating N-cadherin via non-canonical SMAD2/3 signaling.Cell Death Dis. 2018 Feb 7;9(2):174. doi: 10.1038/s41419-017-0230-1. Cell Death Dis. 2018. PMID: 29416020 Free PMC article.
-
Human trophoblast stem cells: Real or not real?Placenta. 2017 Dec;60 Suppl 1(Suppl 1):S57-S60. doi: 10.1016/j.placenta.2017.01.003. Epub 2017 Jan 5. Placenta. 2017. PMID: 28087122 Free PMC article. Review.
-
Human trophoblast invasion: new and unexpected routes and functions.Histochem Cell Biol. 2018 Oct;150(4):361-370. doi: 10.1007/s00418-018-1699-0. Epub 2018 Jul 26. Histochem Cell Biol. 2018. PMID: 30046889 Free PMC article. Review.
Cited by
-
Expression profiling of lncRNAs and mRNAs in placental site trophoblastic tumor (PSTT) by microarray.Int J Med Sci. 2022 Jan 1;19(1):1-12. doi: 10.7150/ijms.65002. eCollection 2022. Int J Med Sci. 2022. PMID: 34975294 Free PMC article.
-
Unraveling the molecular mechanisms driving enhanced invasion capability of extravillous trophoblast cells: a comprehensive review.J Assist Reprod Genet. 2024 Mar;41(3):591-608. doi: 10.1007/s10815-024-03036-6. Epub 2024 Feb 5. J Assist Reprod Genet. 2024. PMID: 38315418 Free PMC article. Review.
-
Anti-Müllerian hormone treatment enhances oocyte quality, embryonic development and live birth rate†.Biol Reprod. 2022 Sep 12;107(3):813-822. doi: 10.1093/biolre/ioac116. Biol Reprod. 2022. PMID: 35657015 Free PMC article.
-
Supraphysiological Role of Melatonin Over Vascular Dysfunction of Pregnancy, a New Therapeutic Agent?Front Physiol. 2021 Nov 16;12:767684. doi: 10.3389/fphys.2021.767684. eCollection 2021. Front Physiol. 2021. PMID: 34867473 Free PMC article. Review.
-
Dysregulated BMP2 in the Placenta May Contribute to Early-Onset Preeclampsia by Regulating Human Trophoblast Expression of Extracellular Matrix and Adhesion Molecules.Front Cell Dev Biol. 2021 Dec 14;9:768669. doi: 10.3389/fcell.2021.768669. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34970543 Free PMC article.
References
-
- Bai S. X., Wang Y. L., Qin L., Xiao Z. J., Herva R., Piao Y. S. (2005). Dynamic expression of matrix metalloproteinases (MMP-2, -9 and -14) and the tissue inhibitors of MMPs (TIMP-1, -2 and -3) at the implantation site during tubal pregnancy. Reproduction 129 103–113. 10.1530/rep.1.00283 - DOI - PubMed
-
- Bartel D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116 281–297. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

