Monocyte/Macrophage Lineage Cells From Fetal Erythromyeloid Progenitors Orchestrate Bone Remodeling and Repair
- PMID: 33614650
- PMCID: PMC7889961
- DOI: 10.3389/fcell.2021.622035
Monocyte/Macrophage Lineage Cells From Fetal Erythromyeloid Progenitors Orchestrate Bone Remodeling and Repair
Abstract
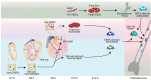
A third of the population sustains a bone fracture, and the pace of fracture healing slows with age. The slower pace of repair is responsible for the increased morbidity in older individuals who sustain a fracture. Bone healing progresses through overlapping phases, initiated by cells of the monocyte/macrophage lineage. The repair process ends with remodeling. This last phase is controlled by osteoclasts, which are bone-specific multinucleated cells also of the monocyte/macrophage lineage. The slower rate of healing in aging can be rejuvenated by macrophages from young animals, and secreted proteins from macrophage regulate undifferentiated mesenchymal cells to become bone-forming osteoblasts. Macrophages can derive from fetal erythromyeloid progenitors or from adult hematopoietic progenitors. Recent studies show that fetal erythromyeloid progenitors are responsible for the osteoclasts that form the space in bone for hematopoiesis and the fetal osteoclast precursors reside in the spleen postnatally, traveling through the blood to participate in fracture repair. Differences in secreted proteins between macrophages from old and young animals regulate the efficiency of osteoblast differentiation from undifferentiated mesenchymal precursor cells. Interestingly, during the remodeling phase osteoclasts can form from the fusion between monocyte/macrophage lineage cells from the fetal and postnatal precursor populations. Data from single cell RNA sequencing identifies specific markers for populations derived from the different precursor populations, a finding that can be used in future studies. Here, we review the diversity of macrophages and osteoclasts, and discuss recent finding about their developmental origin and functions, which provides novel insights into their roles in bone homeostasis and repair.
Keywords: erythromyeloid progenitors; fracture; macrophage; osteoclast; remodeling; yolk sac.
Copyright © 2021 Yahara, Ma, Gracia and Alman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair.Nat Cell Biol. 2020 Jan;22(1):49-59. doi: 10.1038/s41556-019-0437-8. Epub 2020 Jan 6. Nat Cell Biol. 2020. PMID: 31907410 Free PMC article.
-
Early hematopoiesis and macrophage development.Semin Immunol. 2015 Dec;27(6):379-87. doi: 10.1016/j.smim.2016.03.013. Epub 2016 Mar 25. Semin Immunol. 2015. PMID: 27021646 Free PMC article. Review.
-
Interleukin-3 plays dual roles in osteoclastogenesis by promoting the development of osteoclast progenitors but inhibiting the osteoclastogenic process.Biochem Biophys Res Commun. 2013 Nov 1;440(4):545-50. doi: 10.1016/j.bbrc.2013.09.098. Epub 2013 Oct 5. Biochem Biophys Res Commun. 2013. PMID: 24103757 Free PMC article.
-
The Role Of BMPs in the Regulation of Osteoclasts Resorption and Bone Remodeling: From Experimental Models to Clinical Applications.Front Immunol. 2022 Apr 26;13:869422. doi: 10.3389/fimmu.2022.869422. eCollection 2022. Front Immunol. 2022. PMID: 35558080 Free PMC article. Review.
-
The origins and roles of osteoclasts in bone development, homeostasis and repair.Development. 2022 Apr 15;149(8):dev199908. doi: 10.1242/dev.199908. Epub 2022 May 3. Development. 2022. PMID: 35502779 Free PMC article. Review.
Cited by
-
Dual-Functional Drug Delivery System for Bisphosphonate-Related Osteonecrosis Prevention and Its Bioinspired Releasing Model and In Vitro Assessment.ACS Omega. 2023 Jul 14;8(29):26561-26576. doi: 10.1021/acsomega.3c03440. eCollection 2023 Jul 25. ACS Omega. 2023. PMID: 37521598 Free PMC article.
-
Cellular Uptake of Modified Mesoporous Bioactive Glass Nanoparticles for Effective Intracellular Delivery of Therapeutic Agents.Int J Nanomedicine. 2023 Mar 28;18:1599-1612. doi: 10.2147/IJN.S397297. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37013026 Free PMC article.
-
Biocompatible reduced graphene oxide stimulated BMSCs induce acceleration of bone remodeling and orthodontic tooth movement through promotion on osteoclastogenesis and angiogenesis.Bioact Mater. 2022 Feb 6;15:409-425. doi: 10.1016/j.bioactmat.2022.01.021. eCollection 2022 Sep. Bioact Mater. 2022. PMID: 35386350 Free PMC article.
-
Immunoporosis: Role of Innate Immune Cells in Osteoporosis.Front Immunol. 2021 Aug 5;12:687037. doi: 10.3389/fimmu.2021.687037. eCollection 2021. Front Immunol. 2021. PMID: 34421899 Free PMC article. Review.
-
Babam2 negatively regulates osteoclastogenesis by interacting with Hey1 to inhibit Nfatc1 transcription.Int J Biol Sci. 2022 Jul 11;18(11):4482-4496. doi: 10.7150/ijbs.72487. eCollection 2022. Int J Biol Sci. 2022. PMID: 35864959 Free PMC article.
References
-
- Arai F., Miyamoto T., Ohneda O., Inada T., Sudo T., Brasel K., et al. . (1999). Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med. 190, 1741–1754. 10.1084/jem.190.12.1741 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

