Why Is COVID-19 More Severe in Patients With Diabetes? The Role of Angiotensin-Converting Enzyme 2, Endothelial Dysfunction and the Immunoinflammatory System
- PMID: 33614744
- PMCID: PMC7886785
- DOI: 10.3389/fcvm.2020.629933
Why Is COVID-19 More Severe in Patients With Diabetes? The Role of Angiotensin-Converting Enzyme 2, Endothelial Dysfunction and the Immunoinflammatory System
Abstract
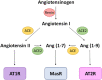
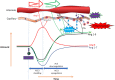
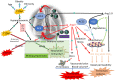
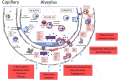
Meta-analyses have indicated that individuals with type 1 or type 2 diabetes are at increased risk of suffering a severe form of COVID-19 and have a higher mortality rate than the non-diabetic population. Patients with diabetes have chronic, low-level systemic inflammation, which results in global cellular dysfunction underlying the wide variety of symptoms associated with the disease, including an increased risk of respiratory infection. While the increased severity of COVID-19 amongst patients with diabetes is not yet fully understood, the common features associated with both diseases are dysregulated immune and inflammatory responses. An additional key player in COVID-19 is the enzyme, angiotensin-converting enzyme 2 (ACE2), which is essential for adhesion and uptake of virus into cells prior to replication. Changes to the expression of ACE2 in diabetes have been documented, but they vary across different organs and the importance of such changes on COVID-19 severity are still under investigation. This review will examine and summarise existing data on how immune and inflammatory processes interplay with the pathogenesis of COVID-19, with a particular focus on the impacts that diabetes, endothelial dysfunction and the expression dynamics of ACE2 have on the disease severity.
Keywords: COVID-19; SARS– CoV– 2; angiotensin converting enzyme-2; diabetes; endothelium; immune response; inflammation; oxidative stress.
Copyright © 2021 Roberts, Pritchard, Treweeke, Rossi, Brace, Cahill, MacRury, Wei and Megson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

