Duration of Dual Antiplatelet Therapy in Patients With Acute Coronary Syndrome Treated With New Generation Stents: A Meta-Analysis of Randomized Controlled Trials
- PMID: 33614748
- PMCID: PMC7886789
- DOI: 10.3389/fcvm.2021.615396
Duration of Dual Antiplatelet Therapy in Patients With Acute Coronary Syndrome Treated With New Generation Stents: A Meta-Analysis of Randomized Controlled Trials
Abstract
Background and Objective: The optimum duration of dual antiplatelet therapy (DAPT) remains uncertain in patients with acute coronary syndrome treated with new generation stents. This meta-analysis was performed to investigate ischemia and bleeding outcomes with different DAPT strategies. Methods: PubMed, Embase, Cochrane and Web of science from inception to May 27, 2020, were systematically searched. Randomized controlled trials were included to compare short-term (6 months or less) with standard (12 months) DAPT in patients with acute coronary syndrome treated with new generation stents. The primary endpoints were myocardial infarction, definite or probable stent thrombosis and major bleeding. The secondary endpoints included all-cause death, cardiovascular death, stroke, target vessel revascularization and net adverse clinical events. Random effect model and fixed effect model were used to calculate the odds ratio (OR) and 95% confidence interval (CI) of each endpoint. Results: Four randomized controlled trials and seven subgroup analyses of larger randomized controlled trials, including a total of 21,344 patients with acute coronary syndrome, met our inclusion criteria. The shorter DAPT was associated with significantly lower major bleeding compared with the standard DAPT (OR 0.71, 95% CI 0.56-0.90, P = 0.005, I 2 = 25%), while without increasing the risk of myocardial infarction (OR 1.18, 0.88-1.58, P = 0.28, I 2 = 20%), definite or probable stent thrombosis (OR 1.60, 0.98-2.59, P = 0.06, I 2 = 0%). No significantly difference was observed in the risk of all-cause death (OR 0.96, 0.72-1.27, P = 0.76, I 2 = 2%), cardiovascular death (OR 0.91, 0.62-1.33, P = 0.62, I 2 = 0%), stroke (OR 0.84, 0.54-1.30, P = 0.43, I 2 = 0%), target vessel revascularization (OR 1.14, 0.84-1.55, P = 0.41, I 2 = 8%), and net adverse clinical events (OR 0.93, 0.80-1.07, P = 0.3, I 2 = 18%) between the two groups. Conclusions: In patients with acute coronary syndrome treated with new generation stents, the shorter DAPT leads to a marked reduction in the risk of major bleeding compared with the standard DAPT. This benefit is achieved without increasing the risk of mortality or ischemic outcomes. The study protocol was registered in PROSPERO (CRD42020189871).
Keywords: acute coronary syndrome; dual antiplatelet therapy; duration; new generation stent; percutaneous coronary intervention.
Copyright © 2021 Zhang, Qiao, Guo, Liang, Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
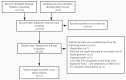

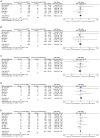
Similar articles
-
Efficacy and safety of short-term 1-3 months versus standard 12 months dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: a meta-analysis of randomized clinical trials.Platelets. 2021 Jul 4;32(5):582-590. doi: 10.1080/09537104.2020.1786039. Epub 2020 Jul 5. Platelets. 2021. PMID: 32627616
-
Efficacy and safety of short-term dual antiplatelet therapy (≤6 months) after percutaneous coronary intervention for acute coronary syndrome: A systematic review and meta-analysis of randomized controlled trials.Clin Cardiol. 2018 Nov;41(11):1455-1462. doi: 10.1002/clc.23075. Epub 2018 Nov 20. Clin Cardiol. 2018. PMID: 30225978 Free PMC article.
-
The optimal discontinuation of dual antiplatelet therapy in patients undergoing percutaneous coronary intervention with drug-eluting stents: A meta-analysis of randomized trials.Int J Cardiol. 2017 May 15;235:73-86. doi: 10.1016/j.ijcard.2017.02.091. Epub 2017 Feb 24. Int J Cardiol. 2017. PMID: 28284499
-
Efficacy and Safety of Very Short-Term Dual Antiplatelet Therapy After Drug-Eluting Stents Implantation for Acute Coronary Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials.Front Cardiovasc Med. 2021 Sep 7;8:660360. doi: 10.3389/fcvm.2021.660360. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34557526 Free PMC article.
-
[Meta-analysis on safety and efficacy of dual antiplatelet therapy combining with proton pump inhibitors for patients after percutaneous coronary intervention].Zhonghua Xin Xue Guan Bing Za Zhi. 2019 Feb 24;47(2):129-140. doi: 10.3760/cma.j.issn.0253-3758.2019.02.010. Zhonghua Xin Xue Guan Bing Za Zhi. 2019. PMID: 30818941 Chinese.
Cited by
-
P2Y12 Antagonists in Cardiovascular Disease-Finding the Best Balance Between Preventing Ischemic Events and Causing Bleeding.Front Cardiovasc Med. 2022 May 12;9:854813. doi: 10.3389/fcvm.2022.854813. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35647068 Free PMC article. Review.
-
Dual antiplatelet therapy after percutaneous coronary intervention in patients at high bleeding risk: A systematic review and meta-analysis.Cardiol J. 2023;30(4):556-566. doi: 10.5603/CJ.a2022.0071. Epub 2022 Aug 1. Cardiol J. 2023. PMID: 35912712 Free PMC article.
-
Antiplatelet Therapy in Low-Platelet-Count Patients After Percutaneous Coronary Intervention for Acute Coronary Syndromes.J Clin Med. 2025 Jan 27;14(3):838. doi: 10.3390/jcm14030838. J Clin Med. 2025. PMID: 39941509 Free PMC article. Review.
References
-
- Mehta SR, Bainey KR, Cantor WJ, Lordkipanidze M, Marquis-Gravel G, Robinson SD, et al. . 2018 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol. (2018) 34:214–33. 10.1016/j.cjca.2017.12.012 - DOI - PubMed
-
- Räber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, et al. . Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. (2012) 125:1110–21. 10.1161/CIRCULATIONAHA.111.058560 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

