Detection of Epithelial Cell Adhesion Molecule in Feline Normal and Tumor Cell Lines and Tissues With Selected Commercial Anti-human EpCAM Antibodies
- PMID: 33614766
- PMCID: PMC7890202
- DOI: 10.3389/fvets.2021.622189
Detection of Epithelial Cell Adhesion Molecule in Feline Normal and Tumor Cell Lines and Tissues With Selected Commercial Anti-human EpCAM Antibodies
Abstract
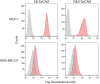
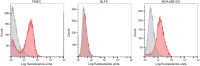
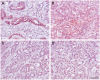
Epithelial cell adhesion molecule (EpCAM) is a transmembrane protein expressed at intercellular junctions in epithelial cells. As an epithelial biomarker, it used for immunologic-based capture of epithelial-derived circulating tumor cells (CTCs) in human patients with different carcinomas. EpCAM expression has not been described in normal or neoplastic epithelial tissues in cats. Our goal was to find a commercial antibody that recognizes surface EpCAM expression for CTC detection. We tested two anti-human EpCAM antibodies, designated for use with flow cytometry, for detection of surface EpCAM expression on feline cell lines derived from normal mammary and renal epithelia and mammary and oropharyngeal squamous cell carcinomas in cats. Only one of the antibodies, a goat polyclonal antibody, labeled normal and neoplastic feline mammary epithelial cells and oropharyngeal squamous cell carcinoma cells; no labeling was observed for normal feline kidney epithelial cells. At low dilution, this antibody immunohistochemically stained the intercellular junctions of normal pancreatic, intestinal and mammary epithelium, as well as neoplastic mammary epithelium in feline tissues; however, oral mucosa, skin, and an oropharyngeal squamous cell carcinoma showed no positive immunostaining. The antibody only weakly bound feline squamous cell carcinoma cell lines under static adhesion. Our results indicate that EpCAM is expressed in specific epithelia in cats but is variably expressed in feline mammary tumors and oropharyngeal squamous cell carcinoma. A higher avidity cross-reactive or feline-specific antibody will be required to further investigate EpCAM expression in normal and neoplastic feline tissue or for detecting CTCs in the blood of tumor-bearing cats.
Keywords: TROP-1/Ep-CAM; cancer; cat; circulating tumor cells; flow cytometry; immunohistochemistry; mammary carcinoma; squamous cell carcinoma.
Copyright © 2021 Heyward, Dong, Shakhzadyan, Wan and Stokol.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Patterns of expression of feline cytokeratins in healthy epithelia and mammary carcinoma cells.Am J Vet Res. 1992 Mar;53(3):304-14. Am J Vet Res. 1992. PMID: 1375818
-
Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors.Int J Oncol. 2005 Jul;27(1):49-57. Int J Oncol. 2005. PMID: 15942643
-
EpCAM expression in squamous cell carcinoma of the uterine cervix detected by monoclonal antibody to the membrane-proximal part of EpCAM.BMC Cancer. 2017 Dec 4;17(1):811. doi: 10.1186/s12885-017-3798-z. BMC Cancer. 2017. PMID: 29202724 Free PMC article.
-
Epithelial cell adhesion molecule expression (CD326) in cancer: a short review.Cancer Treat Rev. 2012 Feb;38(1):68-75. doi: 10.1016/j.ctrv.2011.04.002. Epub 2011 May 14. Cancer Treat Rev. 2012. PMID: 21576002 Review.
-
Shedding light on the EpCAM: An overview.J Cell Physiol. 2019 Aug;234(8):12569-12580. doi: 10.1002/jcp.28132. Epub 2019 Jan 9. J Cell Physiol. 2019. PMID: 30628064 Review.
Cited by
-
A Novel Size-Based Centrifugal Microfluidic Design to Enrich and Magnetically Isolate Circulating Tumor Cells from Blood Cells through Biocompatible Magnetite-Arginine Nanoparticles.Sensors (Basel). 2024 Sep 18;24(18):6031. doi: 10.3390/s24186031. Sensors (Basel). 2024. PMID: 39338775 Free PMC article.
References
-
- Jia M, Mao Y, Wu C, Wang S, Zhang H. A platform for primary tumor origin identification of circulating tumor cells via antibody cocktail-based in vivo capture and specific aptamer-based multicolor fluorescence imaging strategy. Anal Chim Acta. (2019) 1082:136–45. 10.1016/j.aca.2019.07.051 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

