A Multi-Institutional Retrospective Analysis of Toceranib Phosphate for Presumed or Confirmed Canine Aortic Body Chemodectomas
- PMID: 33614771
- PMCID: PMC7892462
- DOI: 10.3389/fvets.2021.635057
A Multi-Institutional Retrospective Analysis of Toceranib Phosphate for Presumed or Confirmed Canine Aortic Body Chemodectomas
Abstract
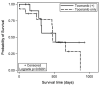
Aortic body tumors, specifically chemodectomas, are the second most common type of canine cardiac tumor; however, information about treatment is currently lacking. This study included dogs with a presumptive or definitive diagnosis of an aortic body chemodectoma that underwent treatment with toceranib phosphate. Cases were solicited via the American College of Veterinary Internal Medicine Cardiology, Internal Medicine, and Oncology listservs using an electronic survey. Cox multivariate analysis of factors potentially impacting survival time was completed. Twenty-seven (27) cases were included in analysis. The clinical benefit rate (complete remission, partial remission, or stable disease >10 weeks) was 89%. A median survival time of 478 days was found for those receiving toceranib alone (n = 14), which was not statistically different from those treated with additional modalities (521 days). No factors evaluated statistically impacted outcome. Further, prospective studies are warranted to evaluate the use of toceranib for the treatment of canine aortic body chemodectomas.
Keywords: Palladia; chemotherapy; dog; heart base tumor; pericardiectomy; radiation.
Copyright © 2021 Coto, Musser, Tropf, Ward, Seo, Mochel and Johannes.
Conflict of interest statement
CJ is a former employee of Pfizer Animal Health. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Withrow SJ, Vail DM, Page RL. Withrow & MacEwen's Small Animal Clinical Oncology. 5th ed St. Louis, Missouri: Elsevier; (2013). p. 750.
-
- Fossum TW. Surgery of the cardiovascular system. In: Small Animal Surgery. 4th ed St. Louis, Mo.: Elsevier Mosby; (2013). p. 896–8.
LinkOut - more resources
Full Text Sources
Other Literature Sources

