The Hsp90 Inhibitor 17-DMAG Attenuates Hyperglycemia-Enhanced Hemorrhagic Transformation in Experimental Stroke
- PMID: 33614785
- PMCID: PMC7878095
- DOI: 10.1155/2021/6668442
The Hsp90 Inhibitor 17-DMAG Attenuates Hyperglycemia-Enhanced Hemorrhagic Transformation in Experimental Stroke
Abstract
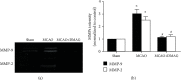
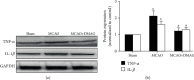
Introduction: Hemorrhagic transformation (HT) is one of the most common complications of ischemic stroke which is exacerbated by hyperglycemia. Oxidative stress, inflammatory response, and matrix metalloproteinases (MMPs) have been evidenced to play a vital role in the pathophysiology of HT. Our previous study has reported that 17-DMAG, an Hsp90 inhibitor, protects the brain against ischemic injury via inhibiting inflammation and reducing MMP-9 after ischemia. However, whether 17-DMAG would attenuate HT in hyperglycemic middle cerebral artery occlusion (MCAO) rats is still unknown.
Methods: Acute hyperglycemia was induced by an injection of 50% dextrose. Rats were pretreated with 17-DMAG before MCAO. Infarction volume, hemorrhagic volume neurological scores, expressions of inflammatory molecules and tight junction proteins, and activity of MMP-2 and MMP-9 were assessed 24 h after MCAO.
Results: 17-DMAG was found to reduce HT, improve neurological function, and inhibit expressions of inflammatory molecules and the activation of MMPs at 24 h after MCAO.
Conclusion: These results implicated that Hsp90 could be a novel therapeutic target in HT following ischemic stroke.
Copyright © 2021 Jiaming Zhang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Hyperbaric oxygen preconditioning attenuates hyperglycemia-enhanced hemorrhagic transformation by inhibiting matrix metalloproteinases in focal cerebral ischemia in rats.Exp Neurol. 2013 Sep;247:737-43. doi: 10.1016/j.expneurol.2013.03.019. Epub 2013 Mar 26. Exp Neurol. 2013. PMID: 23537951 Free PMC article.
-
17-Dimethylaminoethylamino-17-demethoxygeldanamycin attenuates inflammatory responses in experimental stroke.Biol Pharm Bull. 2014;37(11):1713-8. doi: 10.1248/bpb.b14-00208. Biol Pharm Bull. 2014. PMID: 25366476
-
Hyperbaric oxygen preconditioning attenuates hemorrhagic transformation through increasing PPARγ in hyperglycemic MCAO rats.Exp Neurol. 2015 Mar;265:22-9. doi: 10.1016/j.expneurol.2014.12.016. Epub 2014 Dec 24. Exp Neurol. 2015. PMID: 25542160 Free PMC article.
-
Heat shock protein 90 inhibition by 17-DMAG attenuates abdominal aortic aneurysm formation in mice.Am J Physiol Heart Circ Physiol. 2015 Apr 15;308(8):H841-52. doi: 10.1152/ajpheart.00470.2014. Epub 2015 Jan 30. Am J Physiol Heart Circ Physiol. 2015. PMID: 25637544 Free PMC article.
-
Targeted cancer therapy through 17-DMAG as an Hsp90 inhibitor: Overview and current state of the art.Biomed Pharmacother. 2018 Jun;102:608-617. doi: 10.1016/j.biopha.2018.03.102. Epub 2018 Apr 5. Biomed Pharmacother. 2018. PMID: 29602128 Review.
Cited by
-
PAK1 contributes to cerebral ischemia/reperfusion injury by regulating the blood-brain barrier integrity.iScience. 2023 Jul 11;26(8):107333. doi: 10.1016/j.isci.2023.107333. eCollection 2023 Aug 18. iScience. 2023. PMID: 37529106 Free PMC article.
-
Chaperones vs. oxidative stress in the pathobiology of ischemic stroke.Front Mol Neurosci. 2024 Dec 11;17:1513084. doi: 10.3389/fnmol.2024.1513084. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39723236 Free PMC article. Review.
References
-
- Kamada H., Yu F., Nito C., Chan P. H. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007;38(3):1044–1049. doi: 10.1161/01.STR.0000258041.75739.cb. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

