Stress hormones mediate developmental plasticity in vertebrates with complex life cycles
- PMID: 33614863
- PMCID: PMC7879041
- DOI: 10.1016/j.ynstr.2021.100301
Stress hormones mediate developmental plasticity in vertebrates with complex life cycles
Abstract
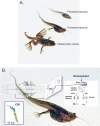
The environment experienced by developing organisms can shape the timing and character of developmental processes, generating different phenotypes from the same genotype, each with different probabilities of survival and performance as adults. Chordates have two basic modes of development, indirect and direct. Species with indirect development, which includes most fishes and amphibians, have a complex life cycle with a free-swimming larva that is typically a growth stage, followed by a metamorphosis into the adult form. Species with direct development, which is an evolutionarily derived developmental mode, develop directly from embryo to the juvenile without an intervening larval stage. Among the best studied species with complex life cycles are the amphibians, especially the anurans (frogs and toads). Amphibian tadpoles are exposed to diverse biotic and abiotic factors in their developmental habitat. They have extensive capacity for developmental plasticity, which can lead to the expression of different, adaptive morphologies as tadpoles (polyphenism), variation in the timing of and size at metamorphosis, and carry-over effects on the phenotype of the juvenile/adult. The neuroendocrine stress axis plays a pivotal role in mediating environmental effects on amphibian development. Before initiating metamorphosis, if tadpoles are exposed to predators they upregulate production of the stress hormone corticosterone (CORT), which acts directly on the tail to cause it to grow, thereby increasing escape performance. When tadpoles reach a minimum body size to initiate metamorphosis they can vary the timing of transformation in relation to growth opportunity or mortality risk in the larval habitat. They do this by modulating the production of thyroid hormone (TH), the primary inducer of metamorphosis, and CORT, which synergizes with TH to promote tissue transformation. Hypophysiotropic neurons that release the stress neurohormone corticotropin-releasing factor (CRF) are activated in response to environmental stress (e.g., pond drying, food restriction, etc.), and CRF accelerates metamorphosis by directly inducing secretion of pituitary thyrotropin and corticotropin, thereby increasing secretion of TH and CORT. Although activation of the neuroendocrine stress axis promotes immediate survival in a deteriorating larval habitat, costs may be incurred such as reduced tadpole growth and size at metamorphosis. Small size at transformation can impair performance of the adult, reducing probability of survival in the terrestrial habitat, or fecundity. Furthermore, elevations in CORT in the tadpole caused by environmental stressors cause long term, stable changes in neuroendocrine function, behavior and physiology of the adult, which can affect fitness. Comparative studies show that the roles of stress hormones in developmental plasticity are conserved across vertebrate taxa including humans.
Keywords: Amphibian; Corticotropin-releasing factor; Developmental plasticity; Glucocorticoid; Metamorphosis; Thyroid hormone.
© 2021 The Author.
Conflict of interest statement
The author, Robert John Denver, declares that he has no conflict of interest.
Figures




Similar articles
-
Lessons from evolution: developmental plasticity in vertebrates with complex life cycles.J Dev Orig Health Dis. 2010 Oct;1(5):282-91. doi: 10.1017/S2040174410000279. J Dev Orig Health Dis. 2010. PMID: 25141931
-
Stress hormones mediate environment-genotype interactions during amphibian development.Gen Comp Endocrinol. 2009 Oct;164(1):20-31. doi: 10.1016/j.ygcen.2009.04.016. Epub 2009 Apr 23. Gen Comp Endocrinol. 2009. PMID: 19393659 Review.
-
Environmental stress as a developmental cue: corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis.Horm Behav. 1997 Apr;31(2):169-79. doi: 10.1006/hbeh.1997.1383. Horm Behav. 1997. PMID: 9154437
-
Endocrine mechanisms underlying plasticity in metamorphic timing in spadefoot toads.Integr Comp Biol. 2003 Nov;43(5):646-57. doi: 10.1093/icb/43.5.646. Integr Comp Biol. 2003. PMID: 21680473
-
Neuroendocrinology of amphibian metamorphosis.Curr Top Dev Biol. 2013;103:195-227. doi: 10.1016/B978-0-12-385979-2.00007-1. Curr Top Dev Biol. 2013. PMID: 23347520 Review.
Cited by
-
Integrating morphology and physiology of the key endocrine organ during tadpole development: The interrenal gland.J Anat. 2022 Dec;241(6):1357-1370. doi: 10.1111/joa.13759. Epub 2022 Sep 2. J Anat. 2022. PMID: 36056596 Free PMC article.
-
The ecological function of thyroid hormones.Philos Trans R Soc Lond B Biol Sci. 2024 Mar 25;379(1898):20220511. doi: 10.1098/rstb.2022.0511. Epub 2024 Feb 5. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38310932 Free PMC article. Review.
-
Stress-induced developmental plasticity and spontaneous preterm birth: A justice-oriented eco-evo-devo review.Eur J Obstet Gynecol Reprod Biol X. 2025 Jun 8;27:100409. doi: 10.1016/j.eurox.2025.100409. eCollection 2025 Sep. Eur J Obstet Gynecol Reprod Biol X. 2025. PMID: 40586095 Free PMC article. Review.
-
Thyroid axis participates in high-temperature-induced male sex reversal through its activation by the stress response.Cell Mol Life Sci. 2023 Aug 17;80(9):253. doi: 10.1007/s00018-023-04913-6. Cell Mol Life Sci. 2023. PMID: 37589787 Free PMC article.
-
Why Causation Matters: Rethinking "Race" as a Risk Factor.Obstet Gynecol. 2023 Oct 1;142(4):766-771. doi: 10.1097/AOG.0000000000005332. Obstet Gynecol. 2023. PMID: 37678936 Free PMC article.
References
-
- Aguilera G. Corticotropin releasing hormone, receptor regulation and the stress response. Trends Endocrinol. Metabol. 1998;9(8):329–336. - PubMed
-
- Alderman S.L., Leishman E.M., Fuzzen M.L.M., Bernier N.J. Corticotropin-releasing factor regulates caspase-3 and may protect developing zebrafish from stress-induced apoptosis. Gen. Comp. Endocrinol. 2018;265:207–213. - PubMed
-
- Altwegg R., Reyer H.U. Patterns of natural selection on size at metamorphosis in water frogs. Evolution. 2003;57(4):872–882. - PubMed
-
- Alvarez D., Nicieza A.G. Effects of induced variation in anuran larval development on postmetamorphic energy reserves and locomotion. Oecologia. 2002;131:186–195. - PubMed
-
- Alyamani R.A.S., Murgatroyd C. Epigenetic programming by early-life stress. In: Grayson D.R., editor. Epigenetics and Psychiatric Disease. 2018. pp. 133–150. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

