Donanemab (LY3002813) dose-escalation study in Alzheimer's disease
- PMID: 33614890
- PMCID: PMC7882532
- DOI: 10.1002/trc2.12112
Donanemab (LY3002813) dose-escalation study in Alzheimer's disease
Abstract
Introduction: This study explored the safety and tolerability features of donanemab (LY3002813) in patients with mild cognitive impairment due to Alzheimer's disease (AD) or mild to moderate AD dementia.
Methods: Patients with AD were enrolled into the single-ascending dose phase and were administered a single, intravenous (IV) dose of donanemab (five dosing cohorts from 0.1 to 10 mg/kg) or placebo followed by a 12-week follow-up period for each dose level. After the follow-up period, the same patients proceeded into the multiple-ascending dose (MAD) phase (five cohorts) and were administered IV doses of donanemab (0.3 to 10 mg/kg) or placebo approximately once per month for up to four doses depending on the initial doses (only cohort 1 went from 0.1 mg/kg to a higher dose of 0.3 mg/kg during the MAD phase). This phase concluded with a 12-week follow-up period. The relative exposure assessment of an unblinded, single, subcutaneous 3-mg/kg dose of donanemab in patients with AD was also performed, followed by a 12-week follow-up period. One cohort of healthy subjects received an unblinded, single, IV 1-mg/kg dose of donanemab. These two cohorts did not continue to the MAD phase.
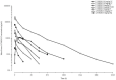
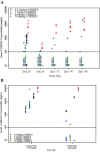
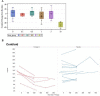
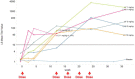
Results: Donanemab was generally well tolerated up to 10 mg/kg. After single-dose administration from 0.1 to 3.0 mg/kg, the mean terminal elimination half-life was ≈4 days, increasing to ≈10 days at 10 mg/kg. Only the 10-mg/kg dose showed changes in amyloid positron emission tomography. Amyloid reduction of 40% to 50% was achieved. Approximately 90% of subjects developed anti-drug antibodies at 3 months after a single intravenous dose.
Discussion: Intravenous donanemab 10 mg/kg can reduce amyloid deposits in AD despite having a shorter than expected half-life.
Keywords: Alzheimer's disease; dementia; donanemab; mild to moderate; tolerability.
© 2020 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals, Inc. on behalf of Alzheimer's Association.
Conflict of interest statement
Authors are employees and minor stockholders of Eli Lilly and Company. Anne Hawdon is a former employee of Eli Lilly and Company.
Figures

References
-
- DeMattos RB, Lu J, Tang Y, et al. A plaque‐specifc antibody clears existing β‐amyloid plaques in Alzheimer's disease mice. Neuron 2012;76(5):908‐920. - PubMed
-
- Barthel H, Gertz H‐J, Dresel S, et al. Florbetaben Study Group . Cerebral amyloid‐β PET with florbetaben (18F) in patients with Alzheimer's disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol 2011;10(5):424‐435. - PubMed
-
- Navitsky M, Joshi AD, Kennedy I, et al. Standardization of amyloid quantitation with florbetapir standardized uptake value ratios to the Centiloid scale. Alzheimers Dement 2018;14(12):1565‐1571. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
