Rate of use and effectiveness of oseltamivir in the treatment of influenza illness in high-risk populations: A systematic review and meta-analysis
- PMID: 33614979
- PMCID: PMC7875571
- DOI: 10.1002/hsr2.241
Rate of use and effectiveness of oseltamivir in the treatment of influenza illness in high-risk populations: A systematic review and meta-analysis
Abstract
Background: Oseltamivir is recommended in the treatment of influenza illness in high-risk populations, including those with chronic heart and lung diseases.
Objectives: We conducted a systematic review and meta-analysis to determine the rate of use and effectiveness of oseltamivir in these groups of patients.
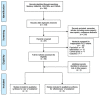
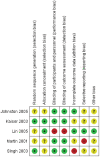
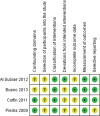
Methods: The protocol for the systematic review was registered on PROSPERO (CRD42019125998). Medline, EMBASE, Cochrane CENTRAL, and CINAHL were searched for observational studies and randomized controlled trials published up to 16 February 2020. Quality appraisal of final studies was conducted using GRADE guidelines. Data were extracted using a predeveloped template. Main outcomes measured included the rate of use of oseltamivir for influenza-like-illness and its effectiveness in reducing disease severity in patients with cardiopulmonary diseases. Outcomes measured for effectiveness were influenza-related complications (respiratory infections and asthma exacerbations), hospitalization rates, and time to freedom from illness. Risk of bias was assessed using Cochrane's Risk of Bias 2.0 tool for randomized trials and Cochrane's Risk of Bias in nonrandomized Studies of Interventions tool for nonrandomized trials. Where data were available, pooled analyses were conducted. Dichotomous variables were evaluated using the Mantel-Hansel method. A random effect model was applied. Summary measures were reported as risk ratios where relevant.
Results: Our systematic review identified nine studies. Oseltamivir use ranged from 25% to 100%. When oseltamivir group was compared to placebo, rates of respiratory tract infections reduced by 28% (RR = 0.72, 95% CI = 0.59-0.90), hospitalization reduced by 52% (RR = 0.48, 95% CI = 0.28-0.80) and median time to illness alleviation decreased by 10.4 to 120 hours. There was no significant reduction in asthma exacerbation rates.
Conclusions: Our systematic review suggests that the use of oseltamivir is beneficial in reducing disease severity, however, its use in high-risk population remains suboptimal.
Keywords: heart diseases; human; influenza; lung diseases; oseltamivir.
© 2021 The Authors. Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



References
-
- WHO . Ask the expert: Influenza Q&A; 2018. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
-
- WHO . WHO Guidelines for Pharmacological Management of Pandemic Influenza A(H1N1) 2009 and other Influenza Viruses. 2010. - PubMed
-
- Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta‐analysis. Lancet. 2011;378(9807):1917‐1930. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

