Amphidinol 3 preferentially binds to cholesterol in disordered domains and disrupts membrane phase separation
- PMID: 33614998
- PMCID: PMC7881217
- DOI: 10.1016/j.bbrep.2021.100941
Amphidinol 3 preferentially binds to cholesterol in disordered domains and disrupts membrane phase separation
Abstract
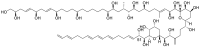
Amphidinol 3 (AM3), a polyhydroxy-polyene metabolite from the dinoflagellate Amphidinium klebsii, possesses potent antifungal activity. AM3 is known to interact directly with membrane sterols and permeabilize membranes by forming pores. Because AM3 binds to sterols such as cholesterol and ergosterol, it can be assumed that AM3 has some impact on lipid rafts, which are membrane domains rich in sphingolipids and cholesterol. Hence, we first examined the effect of AM3 on phase-separated liposomes, in which raft-like ordered and non-raft-like disordered domains are segregated. Consequently, AM3 disrupted the phase separation at 22 μM, as in the case of methyl-β-cyclodextrin, a well-known raft-disrupter that extracts sterol from membranes. The surface plasmon resonance measurements and dye leakage assays show that AM3 preferentially recognizes cholesterol in the disordered membrane, which may reflect a weaker lipid-cholesterol interaction in disordered membrane than in ordered membrane. Finally, to gain insight into the AM3-induced coalescence of membrane phases, we measured membrane fluidity using fluorescence correlation spectroscopy, demonstrating that AM3 significantly increases the order of disordered phase. Together, AM3 preferentially binds to the disordered phase rather than the ordered phase, and enhances the order of the disordered phase, consequently blending the separated phases.
Keywords: Amphidinol 3; Cholesterol; Fluorescence microscope; Lipid rafts; Membrane; Phase separation.
© 2021 The Authors.
Conflict of interest statement
We do not have any conflict of interest.
Figures






Similar articles
-
Direct and stereospecific interaction of amphidinol 3 with sterol in lipid bilayers.Biochemistry. 2014 May 27;53(20):3287-93. doi: 10.1021/bi5002932. Epub 2014 May 12. Biochemistry. 2014. PMID: 24773476
-
Membrane permeabilizing action of amphidinol 3 and theonellamide A in raft-forming lipid mixtures.Z Naturforsch C J Biosci. 2017 Jan 1;72(1-2):43-48. doi: 10.1515/znc-2016-0043. Z Naturforsch C J Biosci. 2017. PMID: 27159918
-
Membrane-permeabilizing activities of amphidinol 3, polyene-polyhydroxy antifungal from a marine dinoflagellate.Biochim Biophys Acta. 2004 Nov 17;1667(1):91-100. doi: 10.1016/j.bbamem.2004.09.002. Biochim Biophys Acta. 2004. PMID: 15533309
-
How principles of domain formation in model membranes may explain ambiguities concerning lipid raft formation in cells.Biochim Biophys Acta. 2005 Dec 30;1746(3):203-20. doi: 10.1016/j.bbamcr.2005.09.002. Epub 2005 Sep 20. Biochim Biophys Acta. 2005. PMID: 16225940 Review.
-
Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications.Subcell Biochem. 2004;37:167-215. doi: 10.1007/978-1-4757-5806-1_5. Subcell Biochem. 2004. PMID: 15376621 Review.
Cited by
-
A Strategy for Gene Knockdown in Dinoflagellates.Microorganisms. 2022 May 31;10(6):1131. doi: 10.3390/microorganisms10061131. Microorganisms. 2022. PMID: 35744649 Free PMC article.
-
A Toxic Sterolysin From a 1950s Culture of Gymnodinium Veneficum Ballantine.Res Sq [Preprint]. 2024 Mar 25:rs.3.rs-3970188. doi: 10.21203/rs.3.rs-3970188/v1. Res Sq. 2024. Update in: Sci Rep. 2024 Aug 3;14(1):17998. doi: 10.1038/s41598-024-68669-0. PMID: 38585775 Free PMC article. Updated. Preprint.
-
Marine natural products targeting the eukaryotic cell membrane.J Antibiot (Tokyo). 2021 Nov;74(11):769-785. doi: 10.1038/s41429-021-00468-5. Epub 2021 Sep 7. J Antibiot (Tokyo). 2021. PMID: 34493848 Review.
-
Sterolysin from a 1950s culture of Karlodinium veneficum (aka Gymnodinium veneficum Ballantine) forms lethal sterol dependent membrane pores.Sci Rep. 2024 Aug 3;14(1):17998. doi: 10.1038/s41598-024-68669-0. Sci Rep. 2024. PMID: 39097621 Free PMC article.
References
-
- Satake M., Murata M., Yasumoto T., Fujita T., Naoki H. Amphidinol, a polyhydroxy-polyene antifungal agent with an unprecedented structure, from a marine dinoflagellate, Amphidinium klebsii. J. Am. Chem. Soc. 1991;113:9859–9861.
-
- Paul G.K., Matsumori N., Murata M., Tachibana K. Isolation and chemical structure of amphidinol 2, a potent hemolytic compound from marine dinoflagellate Amphidinium klebsii. Tetrahedron Lett. 1995;36:6279–6282.
-
- Paul G.K., Matsumori N., Konoki K., Murata M., Tachibana K. Chemical structures of amphidinols 5 and 6 isolated from marine dinoflagellate Amphidinium klebsii and their cholesterol-dependent membrane disruption. J. Mar. Biotechnol. 1997;5:124–128.
-
- Paul G.K., Matsumori N., Konoki K., Sasaki M., Murata M., Tachibana K. Structure of amphidinol 3 and its cholesterol-dependent membrane perturbation: a strong antifungal metabolite produced by dinoflagellate Amphidinium klebsii. In: Yasumoto T., Oshima Y., Fukuyo Y., editors. Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO; Paris: 1996. pp. 503–506.
-
- Murata M., Matsuoka S., Matsumori N., Paul G.K., Tachibana K. Absolute configuration of amphidinol 3, the first complete structure determination from amphidinol homologues: application of a new configuration analysis based on carbon-hydrogen spin-coupling constants. J. Am. Chem. Soc. 1999;121:870–871.
LinkOut - more resources
Full Text Sources
Other Literature Sources

