Recessive Mutations in SYNPO2 as a Candidate of Monogenic Nephrotic Syndrome
- PMID: 33615072
- PMCID: PMC7879128
- DOI: 10.1016/j.ekir.2020.10.040
Recessive Mutations in SYNPO2 as a Candidate of Monogenic Nephrotic Syndrome
Abstract
Introduction: Most of the approximately 60 genes that if mutated cause steroid-resistant nephrotic syndrome (SRNS) are highly expressed in the glomerular podocyte, rendering SRNS a "podocytopathy."
Methods: We performed whole-exome sequencing (WES) in 1200 nephrotic syndrome (NS) patients.
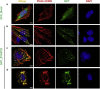
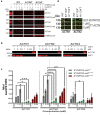
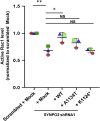
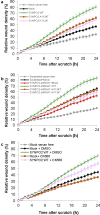
Results: We discovered homozygous truncating and homozygous missense mutation in SYNPO2 (synaptopodin-2) (p.Lys1124∗ and p.Ala1134Thr) in 2 patients with childhood-onset NS. We found SYNPO2 expression in both podocytes and mesangial cells; however, notably, immunofluorescence staining of adult human and rat kidney cryosections indicated that SYNPO2 is localized mainly in mesangial cells. Subcellular localization studies reveal that in these cells SYNPO2 partially co-localizes with α-actinin and filamin A-containing F-actin filaments. Upon transfection in mesangial cells or podocytes, EGFP-SYNPO2 co-localized with α-actinin-4, which gene is mutated in autosomal dominant SRNS in humans. SYNPO2 overexpression increases mesangial cell migration rate (MMR), whereas shRNA knockdown reduces MMR. Decreased MMR was rescued by transfection of wild-type mouse Synpo2 cDNA but only partially by cDNA representing mutations from the NS patients. The increased mesangial cell migration rate (MMR) by SYNPO2 overexpression was inhibited by ARP complex inhibitor CK666. SYNPO2 shRNA knockdown in podocytes decreased active Rac1, which was rescued by transfection of wild-type SYNPO2 cDNA but not by cDNA representing any of the 2 mutant variants.
Conclusion: We show that SYNPO2 variants may lead to Rac1-ARP3 dysregulation, and may play a role in the pathogenesis of nephrotic syndrome.
Keywords: SYNPO2; monogenic kidney disease; nephrotic syndrome.
© 2020 International Society of Nephrology. Published by Elsevier Inc.
Figures


References
-
- Wiggins R.C. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

