Anti-phospholipid syndrome and COVID-19 thrombosis: connecting the dots
- PMID: 33615129
- PMCID: PMC7882149
- DOI: 10.1093/rap/rkaa081
Anti-phospholipid syndrome and COVID-19 thrombosis: connecting the dots
Abstract
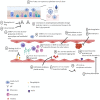
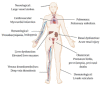
As the coronavirus disease 2019 (COVID-19) pandemic, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is spreading rapidly worldwide, it has emerged as a leading cause of mortality, resulting in >1 million deaths over the past 10 months. The pathophysiology of COVID-19 remains unclear, posing a great challenge to the medical management of patients. Recent studies have reported an unusually high prevalence of thromboembolic events in COVID-19 patients, although the mechanism remains elusive. Several studies have reported the presence of aPLs in COVID-19 patients. We have noticed similarities between COVID-19 and APS, which is an autoimmune prothrombotic disease that is often associated with an infective aetiology. Molecular mimicry and endothelial dysfunction could plausibly explain the mechanism of thrombogenesis in acquired APS. In this review, we discuss the clinicopathological similarities between COVID-19 and APS, and the potential role of therapeutic targets based on the anti-phospholipid model for COVID-19 disease.
Keywords: COVID-19; anti-phospholipid antibodies; anti-phospholipid syndrome; anticoagulation; autoimmunity; molecular mimicry; oxidative stress; thrombosis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
References
-
- World Health Organization. Coronavirus (COVID-19) events as they happen. World Health Organization, 2020. https://covid19.who.int/table.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
