Benefits of early aggressive immunomodulatory therapy (tocilizumab and methylprednisolone) in COVID-19: Single center cohort study of 685 patients
- PMID: 33615209
- PMCID: PMC7879932
- DOI: 10.1016/j.jtauto.2021.100086
Benefits of early aggressive immunomodulatory therapy (tocilizumab and methylprednisolone) in COVID-19: Single center cohort study of 685 patients
Abstract
Introduction: A growing evidence suggests that immune dysregulation and thrombotic phenomena are key features in the pathophysiology of COVID-19. Apart from antivirals and respiratory support, anticoagulants, corticoids and immunomodulators are increasingly being prescribed, especially for more severe cases. We describe the clinical outcome of a large cohort of patients preferentially treated with glucocorticoids and interleukin inhibitors.
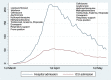
Methods: Single center and retrospective case series. Adult patients admitted with COVID-19 related respiratory insufficiency were included. Patients who died within 2 days after admission and those testing positive but asymptomatic were excluded. We defined two study periods: from March 3rd to March 31 st, 2020 (beginning of epidemic until peak of incidence) and April 1 st to May 7 th, 2020 (second half of epidemic). The majority of patients received respiratory support, combinations of antimicrobials, anticoagulants, corticoids and interleukin inhibitors. Antivirals were preferentially given in the first period. The clinical outcome (death and ventilator dependency) of both periods was compared.
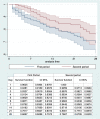
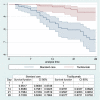
Results: From March 3 rd to May 7 th, 685 patients were included for analysis (58.4% males, mean age 68.9 years). Patients in the first period (n = 408) were younger (66.6 vs 71.1 years, p = 0.003), presented lower mean P a O 2/F i O2 ratio at admission (256.5 vs 270.4 mm Hg,p = 0.0563), higher ferritin (1520 vs 1221 ng/ml, p = 0.01), higher IL-6 (679 vs 194 pg/ml, p < 0.0001) and similar D-dimer levels (3.59 vs 3.39 μg/mL, p = 0.65) compared to the second period (n = 277). Lopinavir/ritonavir and interferon were preferentially given in the first period (23.8% and 32% vs 1.8% and 11.9%, p < 0.0001). Use of corticoids (88.2% vs 87.4%, p = 0,74) and tocilizumab (26.29 vs 20.22% p = 0.06) were similarly administered in both periods. Patients in the second period needed less mechanical ventilation (4.9% vs 16.9%, p < 0.0001), fewer ICU admission (6.1% vs 20.1%,p < 0.0001) and showed similar mortality (17.7% vs 15.4%, p = 0.43). Infectious and thrombotic complications were comparable in both periods (both around 8%, with no statistical difference). Patients treated with tocilizumab (n = 163) had lower mortality rate compared to those untreated under the same indication (7.9% vs 24.2%, p < 0.0001).
Conclusions: In this large retrospective COVID-19 in-hospital cohort, lopinavir/ritonavir and interferon showed no significant impact on survival. Extensive use of corticosteroids and tocilizumab resulted in good overall outcome and showed acceptable complication rates.
Keywords: COVID-19; Coronavirus; Diagnosis; Epidemiology; Immunomodulation; SARS-CoV-2; Treatment.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- WHO coronavirus disease (COVID-19) https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

