Association of plasma cell-free DNA with survival in patients with IDH wild-type glioblastoma
- PMID: 33615225
- PMCID: PMC7883768
- DOI: 10.1093/noajnl/vdab011
Association of plasma cell-free DNA with survival in patients with IDH wild-type glioblastoma
Abstract
Background: We aimed to determine whether plasma cell-free DNA (cfDNA) concentration is associated with survival in patients with isocitrate dehydrogenase (IDH) wild-type glioblastoma (GBM).
Methods: Pre-operative and post-chemoradiotherapy blood samples were prospectively collected from patients with newly diagnosed IDH wild-type GBM. Patients underwent surgical resection or biopsy and received adjuvant radiotherapy with concomitant temozolomide. Cell-free DNA (cfDNA) was isolated from plasma and quantified using SYBR Green-based q polymerase chain reaction (qPCR).
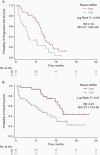
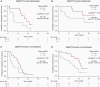
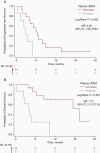
Results: Sixty-two patients were enrolled and categorized into high vs. low cfDNA groups relative to the pre-operative median value (25.2 ng/mL, range 5.7-153.0 ng/mL). High pre-operative cfDNA concentration was associated with inferior PFS (median progression-free survival (PFS), 3.4 vs. 7.7 months; log-rank P = .004; hazard ratio [HR], 2.19; 95% CI, 1.26-3.81) and overall survival (OS) (median OS, 8.0 vs. 13.9 months; log-rank P = .01; HR, 2.43; 95% CI, 1.19-4.95). After adjusting for risk factors, including O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status, pre-operative cfDNA remained independently associated with PFS (HR, 2.70; 95% CI, 1.50-4.83; P = .001) and OS (HR, 2.65; 95% CI, 1.25-5.59; P = .01). Post-hoc analysis of change in cfDNA post-chemoradiotherapy compared to pre-surgery (n = 24) showed increasing cfDNA concentration was associated with worse PFS (median, 2.7 vs. 6.0 months; log-rank P = .003; HR, 4.92; 95% CI, 1.53-15.84) and OS (median, 3.9 vs. 19.4 months; log-rank P < .001; HR, 7.77; 95% CI, 2.17-27.76).
Conclusions: cfDNA concentration is a promising prognostic biomarker for patients with IDH wild-type GBM. Plasma cfDNA can be obtained noninvasively and may enable more accurate estimates of survival and effective clinical trial stratification.
Keywords: cell-free DNA; glioblastoma; liquid biopsy; overall survival; progression-free survival.
© The Author(s) 2021. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Figures
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
