The Role of ERO1α in Modulating Cancer Progression and Immune Escape
- PMID: 33615311
- PMCID: PMC7894644
- DOI: 10.33696/cancerimmunol.2.023
The Role of ERO1α in Modulating Cancer Progression and Immune Escape
Abstract
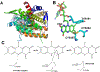
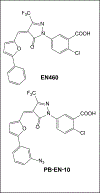
Endoplasmic reticulum oxidoreductin-1 alpha (ERO1α) was originally shown to be an endoplasmic reticulum (ER) resident protein undergoing oxidative cycles in concert with protein disulfide isomerase (PDI) to promote proper protein folding and to maintain homeostasis within the ER. ERO1α belongs to the flavoprotein family containing a flavin adenine dinucleotide utilized in transferring of electrons during oxidation-reduction cycles. This family is used to maintain redox potentials and protein homeostasis within the ER. ERO1α's location and function has since been shown to exist beyond the ER. Originally thought to exist solely in the ER, it has since been found to exist in the golgi apparatus, as well as in exosomes purified from patient samples. Besides aiding in protein folding of transmembrane and secretory proteins in conjunction with PDI, ERO1α is also known for formation of de novo disulfide bridges. Public databases, such as the Cancer Genome Atlas (TCGA) and The Protein Atlas, reveal ERO1α as a poor prognostic marker in multiple disease settings. Recent evidence indicates that ERO1α expression in tumor cells is a critical determinant of metastasis. However, the impact of increased ERO1α expression in tumor cells extends into the tumor microenvironment. Secretory proteins requiring ERO1α expression for proper folding have been implicated as being involved in immune escape through promotion of upregulation of programmed death ligand-1 (PD-L1) and stimulation of polymorphonuclear myeloid derived suppressor cells (PMN-MDSC's) via secretion of granulocytic colony stimulating factor (G-CSF). Hereby, ERO1α plays a pivotal role in cancer progression and potentially immune escape; making ERO1α an emerging attractive putative target for the treatment of cancer.
Keywords: Cancer; Cancer therapeutics; ER stress; ERO1; Immune resistance.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures


References
-
- Abbosh C, Birkbak NJ, Swanton C. Early stage NSCLC—challenges to implementing ctDNA-based screening and MRD detection. Nature Reviews Clinical Oncology. 2018. September;15(9):577–86. - PubMed
-
- Ootsuka S, Asami S, Sasaki T, Yoshida Y, Nemoto N, Shichino H, et al. Useful markers for detecting minimal residual disease in cases of neuroblastoma. Biological and Pharmaceutical Bulletin. 2008. June 1;31(6):1071–4. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
