Visual assessment of [18F]flutemetamol PET images can detect early amyloid pathology and grade its extent
- PMID: 33615397
- PMCID: PMC8175297
- DOI: 10.1007/s00259-020-05174-2
Visual assessment of [18F]flutemetamol PET images can detect early amyloid pathology and grade its extent
Abstract
Purpose: To investigate the sensitivity of visual read (VR) to detect early amyloid pathology and the overall utility of regional VR.
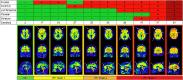
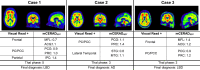
Methods: [18F]Flutemetamol PET images of 497 subjects (ALFA+ N = 352; ADC N = 145) were included. Scans were visually assessed according to product guidelines, recording the number of positive regions (0-5) and a final negative/positive classification. Scans were quantified using the standard and regional Centiloid (CL) method. The agreement between VR-based classification and published CL-based cut-offs for early (CL = 12) and established (CL = 30) pathology was determined. An optimal CL cut-off maximizing Youden's index was derived. Global and regional CL quantification was compared to VR. Finally, 28 post-mortem cases from the [18F]flutemetamol phase III trial were included to assess the percentage agreement between VR and neuropathological classification of neuritic plaque density.
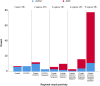
Results: VR showed excellent agreement against CL = 12 (κ = .89, 95.2%) and CL = 30 (κ = .88, 95.4%) cut-offs. ROC analysis resulted in an optimal CL = 17 cut-off against VR (sensitivity = 97.9%, specificity = 97.8%). Each additional positive VR region corresponded to a clear increase in global CL. Regional VR was also associated with regional CL quantification. Compared to mCERADSOT-based classification (i.e., any region mCERADSOT > 1.5), VR was in agreement in 89.3% of cases, with 13 true negatives, 12 true positives, and 3 false positives (FP). Regional sparse-to-moderate neuritic and substantial diffuse Aβ plaque was observed in all FP cases. Regional VR was also associated with regional plaque density.
Conclusion: VR is an appropriate method for assessing early amyloid pathology and that grading the extent of visual amyloid positivity could present clinical value.
Keywords: Amyloid PET; Centiloid; Neuropathology; Regional visual read; Sensitivity; [18F]flutemetamol.
Conflict of interest statement
Lyduine E. Collij; Gemma Salvadó; Mahnaz Shekari; Isadora Lopes Alves; Juhan Reimand; Alle Meije Wink; Marissa Zwan; Aida Niñerola-Baizán & Andrés Perissinotti all report no existing potential conflicts of interest relevant to this article.
Prof. Philip Scheltens received grants from GE Healthcare, Piramal, and Merck, paid to his institution; he has received speaker’s fees paid to the institution Alzheimer Center, VU University Medical Center, Lilly, GE Healthcare, and Roche.
Prof. Frederik Barkhof received payment and honoraria from Bayer-Schering Pharma, Sanofi-Aventis, Genzyme, Biogen-Idec, TEVA, Merck-Serono, Novartis, Roche, Jansen Research, IXICO Ltd., GeNeuro, and Apitope Ltd. for consulting; payment from the Serono Symposia Foundation, IXICOLtd, and MedScape for educational presentations; research support via grants from EU/EFPIA Innovative Medicines Initiative Joint Undertaking (AMYPAD consortium), EuroPOND (H2020), UK MS Society, Dutch MS Society, PICTURE (IMDI-NWO), NIHR UCLH Biomedical Research Centre (BRC), ECTRIMS-MAGNIMS.
Prof. J. L. Molinuevo is currently a full-time employee of Lundbeck and priory has served as a consultant or at advisory boards for the following for-profit companies, or has given lectures in symposia sponsored by the following for-profit companies: Roche Diagnostics, Genentech, Novartis, Lundbeck, Oryzon, Biogen, Lilly, Janssen, Green Valley, MSD, Eisai, Alector, BioCross, GE Healthcare, ProMIS Neurosciences. He also received research support from the EU/EFPIA Innovative Medicines Initiative Joint Undertaking AMYPAD grant agreement n° 115952; the EU/EFPIA Innovative Medicines Initiative Joint Undertaking EPAD grant agreement n° 115736; the EU/EFPIA Innovative Medicines Initiative Joint Undertaking AETIONOMY grant n° 115568; and ‘la Caixa’ Foundation.
Dr. J.D. Gispert has received speaker’s fees from Biogen and Philips. In addition he holds a ‘Ramón y Cajal’ fellowship (RYC-2013-13054) from the Spanish Ministry of Economy and Competitiveness, has received research support from the EU/EFPIA Innovative Medicines Initiative Joint Undertaking AMYPAD grant agreement n° 115952, and from Ministerio de Ciencia y Universidades (grant agreement RTI2018-102261).
Prof. BN.M. van Berckel received research support from ZON-MW, AVID radiopharmaceuticals, CTMM and Janssen Pharmaceuticals. BvB is a trainer for Piramal and GE; he receives no personal honoraria.
G Farrar, C Buckley and APL Smith are full-time employees of GE Healthcare.
MD Ikonomovic has received research funding from GE Healthcare.
No other potential conflicts of interest relevant to this article exist.
Figures



References
-
- Salloway S, Gamez JE, Singh U, Sadowsky CH, Villena T, Sabbagh MN, et al. Performance of [(18)F]flutemetamol amyloid imaging against the neuritic plaque component of CERAD and the current (2012) NIA-AA recommendations for the neuropathologic diagnosis of Alzheimer's disease. Alzheimers Dement (Amst) 2017;9:25–34. doi: 10.1016/j.dadm.2017.06.001. - DOI - PMC - PubMed
-
- Thal DR, Beach TG, Zanette M, Heurling K, Chakrabarty A, Ismail A, et al. [(18)F]flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer's disease: specific detection of advanced phases of amyloid-beta pathology. Alzheimers Dement. 2015;11:975–985. doi: 10.1016/j.jalz.2015.05.018. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

