First-Line Biological Agents Plus Chemotherapy in Older Patients with Metastatic Colorectal Cancer: A Retrospective Pooled Analysis
- PMID: 33615402
- PMCID: PMC7914239
- DOI: 10.1007/s40266-021-00834-w
First-Line Biological Agents Plus Chemotherapy in Older Patients with Metastatic Colorectal Cancer: A Retrospective Pooled Analysis
Abstract
Background: Biologicals, in combination with chemotherapy, are recommended as first-line treatment of metastatic colorectal cancer (mCRC); however, evidence guiding the appropriate management of older patients with mCRC is limited.
Objective: This study was undertaken to compare the efficacy and safety outcomes in older versus younger patients with mCRC who received first-line biological therapy.
Methods: This retrospective analysis used pooled data from five trials undertaken by the Spanish Cooperative Group for the Treatment of Digestive Tumours. All were studies of adults with advanced CRC who received first-line treatment with chemotherapy plus bevacizumab, cetuximab or panitumumab, stratified by age (≥ 65 vs. < 65 years). Endpoints included progression-free survival (PFS), overall survival (OS), overall response rate (ORR) and safety.
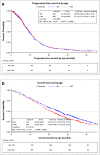
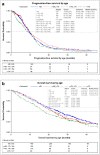
Results: In total, 999 patients from five studies were included in the analysis: 480 (48%) were aged ≥ 65 years, and 519 (52%) were aged < 65 years. Median PFS did not differ significantly between patients aged ≥ 65 and < 65 years (9.9 vs. 9.4 months; hazard ratio [HR] 1.01; 95% confidence interval [CI] 0.88-1.17). Median OS was significantly shorter in older than in younger patients (21.3 vs. 25.0 months; HR 1.21; 95% CI 1.04-1.41). There was no significant difference between older and younger patients in ORR (59 vs. 62%). Patients aged ≥ 65 years experienced significantly more treatment-related grade 3 or higher adverse events (61.67%) than did patients aged < 65 years (45.86%).
Conclusions: Biologicals plus chemotherapy is an effective first-line treatment option for selected patients aged ≥ 65 years with mCRC and has a manageable safety profile and efficacy comparable to that observed in younger patients.
Conflict of interest statement
E Díaz Rubio has received grants from SYSMEX, Merck Serono, Roche, AstraZeneca and Amgen. A Carrato is an advisory board member for Merck, Roche, MSD, Pfizer, Bayer, Shire, Servier, Celgene and Amgen and has received research funds from Celgene, Shire and Amgen. B Massutí has received payment for consulting or advisory roles from Roche, Boehringer Ingelheim, Bristol-Myers Squibb, Merck Sharpe and Dohme and AstraZeneca; participating in speakers' bureau from Roche, Amgen, Merck Serono, Pfizer, AstraZeneca and Boehringer Ingelheim; and travel, accommodation or expenses from Boehringer Ingelheim, Merck Sharp and Dohme, Janssen and Roche. A Muñoz has received grants from Sanofi, Celgene, Leo Pharma; non-financial support from Merck Serono; personal fees and non-financial support from Sanofi, Celgene, Roche and Amgen; and personal fees from AstraZeneca, Leo Pharma, Servier, Pfizer, Daiichi Sankyo, Bayer and Halozyme, Merck Sharp and Dohme, Rovi and Lilly. J Sastre has received honoraria for speaking roles with Merck, Roche, Ipsen, Lilly, Shire, Pfizer, Servier and MSD; advisory roles with Roche, Amgen, Merck, Bayer, Celgene, Servier, BMS, Sanofi and Ipsen; and travelling and accommodation support from Merck and Ipsen. R Ferreiro has received honoraria for speaking and advisory roles and personal fees and travelling and accommodation support from Roche, Amgen and Servier. M Valladares-Ayerbes has received grants from Roche and personal fees from Roche, Merck, Amgen, Sanofi, Servier, Bayer and Celgene. JM Viéitez has received payments for consulting or advisory roles from Roche, Amgen and Servier; travel, accommodation and expenses from Roche, Amgen, Servier and Bristol-Myers Squibb; expert testimony from Servier; and research funding from Amgen and Roche. E Aranda has received honoraria for advisory roles from Amgen, Bayer, Celgene, Merck, Roche and Sanofi. P García Alfonso, A Abad, MJ Ortiz-Morales, JL Manzano Mozo, G Durán, MJ Safont, F Rivera, E González, C Grávalos, V AlonsoOrduña and A Yubero have no conflicts of interest that are directly relevant to the content of this article.
Figures
References
-
- Wilkins T, McMechan D, Talukder A. Colorectal cancer screening and prevention. Am Fam Physician. 2018;97(10):658–665. - PubMed
-
- National Cancer Institute. SEER cancer statistics review, 1975–2016, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. Bethesda, MD. https://seer.cancer.gov/csr/1975_2016/. Accessed 12 June 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

