Treatment tolerability and outcomes in elderly patients with head and neck cancer
- PMID: 33615611
- PMCID: PMC9521799
- DOI: 10.1002/hed.26548
Treatment tolerability and outcomes in elderly patients with head and neck cancer
Abstract
Purpose: The number of elderly patients with head and neck squamous cell carcinoma (HNSCC) continues to grow. Management of this cohort remains poorly defined. We investigated treatment tolerability and clinical outcomes in this underrepresented population.
Methods: We identified patients aged ≥70 with nonrecurrent, nonmetastatic HNSCC treated curatively from 2007-2018 and analyzed clinical covariates.
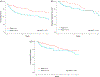
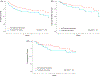
Results: Two hundred and twenty patients with a median age of 75 (interquartile range:72-80) were identified. Age and comorbidities were not correlated with toxicity (P ≥ .05). Patients who experienced a treatment interruption had significantly greater weight loss (P = .042) and worse overall survival (OS) (P < .001), but not worse disease-specific survival (P = .45), or locoregional control (P = .21).
Conclusions: Treatment interruptions were associated with weight loss and worse OS, but not disease related outcomes, suggesting an interruption in the elderly may be a surrogate for another issue. In sum, our data should guide clinical trial design to benefit this growing, neglected cohort.
Keywords: elderly; geriatric; head and neck cancer; squamous cell carcinoma; treatment tolerability.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Figures




Similar articles
-
Clinical and Therapeutic Considerations for Older Adults with Head and Neck Cancer.Clin Interv Aging. 2023 Mar 17;18:409-422. doi: 10.2147/CIA.S366155. eCollection 2023. Clin Interv Aging. 2023. PMID: 36959837 Free PMC article. Review.
-
Radiotherapy for geriatric head-and-neck cancer patients: what is the value of standard treatment in the elderly?Radiat Oncol. 2020 Feb 4;15(1):31. doi: 10.1186/s13014-020-1481-z. Radiat Oncol. 2020. PMID: 32019576 Free PMC article. Clinical Trial.
-
Survival outcomes with concurrent chemoradiation for elderly patients with locally advanced head and neck cancer according to the National Cancer Data Base.Cancer. 2016 May 15;122(10):1533-43. doi: 10.1002/cncr.29956. Epub 2016 Mar 11. Cancer. 2016. PMID: 26969811
-
Definitive chemoradiotherapy in patients with squamous cell cancers of the head and neck - results from an unselected cohort of the clinical cooperation group "Personalized Radiotherapy in Head and Neck Cancer".Radiat Oncol. 2020 Jan 6;15(1):7. doi: 10.1186/s13014-019-1452-4. Radiat Oncol. 2020. PMID: 31906998 Free PMC article.
-
Evolution of treatment and high-risk features in resectable locally advanced Head and Neck squamous cell carcinoma with special reference to extracapsular extension of nodal disease.J BUON. 2015 Jul-Aug;20(4):943-53. J BUON. 2015. PMID: 26416042 Review.
Cited by
-
Adjuvant Reirradiation With Proton Therapy in Head and Neck Squamous Cell Carcinoma.Adv Radiat Oncol. 2023 Dec 10;9(4):101418. doi: 10.1016/j.adro.2023.101418. eCollection 2024 Apr. Adv Radiat Oncol. 2023. PMID: 38778826 Free PMC article.
-
Efficacy evaluation of minimally invasive particle implantation in treating head and neck malignancies under different guidance methods: a propensity score matching analysis study.World J Surg Oncol. 2025 Aug 19;23(1):315. doi: 10.1186/s12957-025-03906-y. World J Surg Oncol. 2025. PMID: 40830976 Free PMC article.
-
Impact of the SARS-CoV-2 pandemic on ophthalmic care in Germany.Ophthalmologe. 2021 Jul;118(Suppl 2):166-175. doi: 10.1007/s00347-021-01411-7. Epub 2021 Jun 4. Ophthalmologe. 2021. PMID: 34086070 Free PMC article.
-
Clinical and Therapeutic Considerations for Older Adults with Head and Neck Cancer.Clin Interv Aging. 2023 Mar 17;18:409-422. doi: 10.2147/CIA.S366155. eCollection 2023. Clin Interv Aging. 2023. PMID: 36959837 Free PMC article. Review.
-
Risk factors and prediction models for severe radiation-induced oral mucositis in patients with nasopharyngeal carcinoma undergoing chemoradiotherapy.Discov Oncol. 2025 May 24;16(1):903. doi: 10.1007/s12672-025-02458-7. Discov Oncol. 2025. PMID: 40411645 Free PMC article.
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA Cancer J Clin. 2002;55(2):74–108. - PubMed
-
- Clayburgh DR. Grandis JR. 3 - molecular biology. In: Bell RB, Fernandes RP, Andersen PE, eds. Oral, Head and Neck Oncology and Reconstructive Surgery. Amsterdam, Netherlands: Elsevier; 2018:79–89.
-
- Sikora AG. The changing demographics of head and neck squamous cell carcinoma in the United States. Laryngoscope. 2004;114(11):1915–1923. - PubMed
-
- SEER Cancer Statistics Review, 1975–2016. National Cancer Institue; 2019. https://seer.cancer.gov/csr/1975_2016/. Accessed August 8, 2019.
-
- Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004; 22(22):4626–4631. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

