Categorizing human vocal signals depends on an integrated auditory-frontal cortical network
- PMID: 33615612
- PMCID: PMC7927295
- DOI: 10.1002/hbm.25309
Categorizing human vocal signals depends on an integrated auditory-frontal cortical network
Abstract
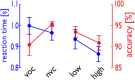
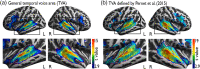
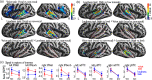
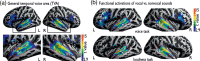
Voice signals are relevant for auditory communication and suggested to be processed in dedicated auditory cortex (AC) regions. While recent reports highlighted an additional role of the inferior frontal cortex (IFC), a detailed description of the integrated functioning of the AC-IFC network and its task relevance for voice processing is missing. Using neuroimaging, we tested sound categorization while human participants either focused on the higher-order vocal-sound dimension (voice task) or feature-based intensity dimension (loudness task) while listening to the same sound material. We found differential involvements of the AC and IFC depending on the task performed and whether the voice dimension was of task relevance or not. First, when comparing neural vocal-sound processing of our task-based with previously reported passive listening designs we observed highly similar cortical activations in the AC and IFC. Second, during task-based vocal-sound processing we observed voice-sensitive responses in the AC and IFC whereas intensity processing was restricted to distinct AC regions. Third, the IFC flexibly adapted to the vocal-sounds' task relevance, being only active when the voice dimension was task relevant. Forth and finally, connectivity modeling revealed that vocal signals independent of their task relevance provided significant input to bilateral AC. However, only when attention was on the voice dimension, we found significant modulations of auditory-frontal connections. Our findings suggest an integrated auditory-frontal network to be essential for behaviorally relevant vocal-sounds processing. The IFC seems to be an important hub of the extended voice network when representing higher-order vocal objects and guiding goal-directed behavior.
Keywords: DCM; auditory-frontal network; decision-making; fMRI; voice.
© 2020 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Stimulus-dependent activations and attention-related modulations in the auditory cortex: a meta-analysis of fMRI studies.Hear Res. 2014 Jan;307:29-41. doi: 10.1016/j.heares.2013.08.001. Epub 2013 Aug 11. Hear Res. 2014. PMID: 23938208 Review.
-
Suppression of irrelevant sounds during auditory working memory.Neuroimage. 2017 Nov 1;161:1-8. doi: 10.1016/j.neuroimage.2017.08.040. Epub 2017 Aug 14. Neuroimage. 2017. PMID: 28818692 Free PMC article.
-
Functional connectivity within the voice perception network and its behavioural relevance.Neuroimage. 2018 Dec;183:356-365. doi: 10.1016/j.neuroimage.2018.08.011. Epub 2018 Aug 9. Neuroimage. 2018. PMID: 30099078 Free PMC article.
-
Auditory cortical micro-networks show differential connectivity during voice and speech processing in humans.Commun Biol. 2021 Jun 25;4(1):801. doi: 10.1038/s42003-021-02328-2. Commun Biol. 2021. PMID: 34172824 Free PMC article.
-
[Auditory perception and language: functional imaging of speech sensitive auditory cortex].Rev Neurol (Paris). 2001 Sep;157(8-9 Pt 1):837-46. Rev Neurol (Paris). 2001. PMID: 11677406 Review. French.
Cited by
-
Effective connectivity underlying reward-based executive control.Hum Brain Mapp. 2021 Oct 1;42(14):4555-4567. doi: 10.1002/hbm.25564. Epub 2021 Jun 26. Hum Brain Mapp. 2021. PMID: 34173997 Free PMC article.
-
Electrocorticography reveals the dynamics of famous voice responses in human fusiform gyrus.J Neurophysiol. 2023 Feb 1;129(2):342-346. doi: 10.1152/jn.00459.2022. Epub 2022 Dec 28. J Neurophysiol. 2023. PMID: 36576268 Free PMC article.
-
Psychopathic and autistic traits differentially influence the neural mechanisms of social cognition from communication signals.Transl Psychiatry. 2022 Nov 29;12(1):494. doi: 10.1038/s41398-022-02260-x. Transl Psychiatry. 2022. PMID: 36446775 Free PMC article.
-
Cortical-striatal brain network distinguishes deepfake from real speaker identity.Commun Biol. 2024 Jun 11;7(1):711. doi: 10.1038/s42003-024-06372-6. Commun Biol. 2024. PMID: 38862808 Free PMC article.
-
Brain mechanism of unfamiliar and familiar voice processing: an activation likelihood estimation meta-analysis.PeerJ. 2023 Mar 13;11:e14976. doi: 10.7717/peerj.14976. eCollection 2023. PeerJ. 2023. PMID: 36935917 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

