B cells modulate mouse allergen-specific T cells in nonallergic laboratory animal-care workers
- PMID: 33616085
- PMCID: PMC7934936
- DOI: 10.1172/jci.insight.145199
B cells modulate mouse allergen-specific T cells in nonallergic laboratory animal-care workers
Abstract
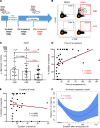
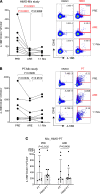
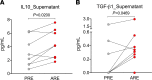
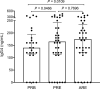
Understanding the mechanisms of allergen-specific immune modulation in nonallergic individuals is key to recapitulate immune tolerance and to develop novel allergy treatments. Herein, we characterized mouse-specific T cell responses in nonallergic laboratory animal-care workers before and after reexposure to mice. PBMCs were collected and stimulated with developed peptide pools identified from high-molecular-weight fractions of mouse allergen extracts. Sizable CD4 T cell responses were noted and were temporarily decreased in most subjects upon reexposure, with the magnitude of decrease positively correlated with time of reexposure but not the duration of the break. Interestingly, the suppression was specific to mouse allergens without affecting responses of bystander antigens. Further, PBMC fractioning studies illustrated that the modulation is unlikely from T cells, while B cell depletion and exchange reversed the suppression of responses, suggesting that B cells may be the key modulators. Increased levels of regulatory cytokines (IL-10 and TGF-β1) in the cell culture supernatant and plasma mouse-specific IgG4 were also observed after reexposure, consistent with B cell-mediated modulation mechanisms. Overall, these results suggest that nonallergic status is achieved by an active, time-related, allergen-specific, B cell-dependent regulatory process upon reexposure, the mechanisms of which should be detailed by further molecular studies.
Keywords: Allergy; Antigen; Immunology; T cells.
Conflict of interest statement
Figures

Similar articles
-
Induction of bystander tolerance and immune deviation after Fel d 1 peptide immunotherapy.J Allergy Clin Immunol. 2019 Mar;143(3):1087-1099.e4. doi: 10.1016/j.jaci.2018.03.023. Epub 2018 Jun 12. J Allergy Clin Immunol. 2019. PMID: 29906527
-
Chronic cat allergen exposure induces a TH2 cell-dependent IgG4 response related to low sensitization.J Allergy Clin Immunol. 2015 Dec;136(6):1627-1635.e13. doi: 10.1016/j.jaci.2015.07.031. Epub 2015 Sep 11. J Allergy Clin Immunol. 2015. PMID: 26371841 Free PMC article.
-
Triggering of specific Toll-like receptors and proinflammatory cytokines breaks allergen-specific T-cell tolerance in human tonsils and peripheral blood.J Allergy Clin Immunol. 2013 Mar;131(3):875-85. doi: 10.1016/j.jaci.2012.10.051. Epub 2012 Dec 23. J Allergy Clin Immunol. 2013. PMID: 23265862
-
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review.
-
T-cell response to allergens.Chem Immunol Allergy. 2010;95:22-44. doi: 10.1159/000315936. Epub 2010 Jun 1. Chem Immunol Allergy. 2010. PMID: 20519880 Review.
Cited by
-
Ex vivo assays show human gamma-delta T cells specific for common allergens are Th1-polarized in allergic donors.Cell Rep Methods. 2022 Nov 21;2(12):100350. doi: 10.1016/j.crmeth.2022.100350. eCollection 2022 Dec 19. Cell Rep Methods. 2022. PMID: 36590684 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

