Morphometric Analysis of Retinal Pigment Epithelial Cells From C57BL/6J Mice During Aging
- PMID: 33616620
- PMCID: PMC7910641
- DOI: 10.1167/iovs.62.2.32
Morphometric Analysis of Retinal Pigment Epithelial Cells From C57BL/6J Mice During Aging
Abstract
Purpose: To quantitatively evaluate the changes in orientation and morphometric features of mouse retinal pigment epithelial (RPE) cells in different regions of the eye during aging.
Methods: We segmented individual RPE cells from whole RPE flatmount images of C57BL/6J mice (postnatal days 30 to 720) using a machine-learning method and evaluated changes in morphometric features, including our newly developed metric combining alignment and shape of RPE cells during aging.
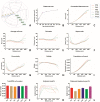
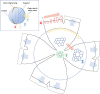
Results: Mainly, the anterior part of the RPE sheet grows during aging, while the posterior part remains constant. Changes in size and shape of the peripheral RPE cells are prominent with aging as cells become larger, elongated, and concave. Conversely, the central RPE cells maintain relatively constant size and numbers with aging. Cell count in the central area and the overall cell count (approximately 50,000) were relatively constant over different age groups. RPE cells also present a specific orientation concordance that matches the shape of the specific region of the eyeball. Those cells near the optic disc or equator have a circumferential orientation to cover the round shape of the eyeball, whereas those cells in the periphery have a radial orientation and corresponding radial elongation, the extent of which increases with aging and matches with axial elongation of the eyeball.
Conclusions: These results suggest that the fluid RPE morphology reflects various growth rates of underlying eyeball, and RPE cells could be classified into four regional classes (near the optic disc, central, equatorial, and peripheral) according to their morphometric features.
Conflict of interest statement
Disclosure:
Figures




Similar articles
-
Methodologies for analysis of patterning in the mouse RPE sheet.Mol Vis. 2015 Jan 15;21:40-60. eCollection 2015. Mol Vis. 2015. PMID: 25593512 Free PMC article.
-
Functional principal component analysis reveals discriminating categories of retinal pigment epithelial morphology in mice.Invest Ophthalmol Vis Sci. 2013 Nov 5;54(12):7274-83. doi: 10.1167/iovs.13-12450. Invest Ophthalmol Vis Sci. 2013. PMID: 24114543 Free PMC article.
-
Retinal pigment epithelial cell multinucleation in the aging eye - a mechanism to repair damage and maintain homoeostasis.Aging Cell. 2016 Jun;15(3):436-45. doi: 10.1111/acel.12447. Epub 2016 Feb 15. Aging Cell. 2016. PMID: 26875723 Free PMC article.
-
Analysis of RPE morphometry in human eyes.Mol Vis. 2016 Jul 30;22:898-916. eCollection 2016. Mol Vis. 2016. PMID: 27555739 Free PMC article.
-
RPE Cell and Sheet Properties in Normal and Diseased Eyes.Adv Exp Med Biol. 2016;854:757-63. doi: 10.1007/978-3-319-17121-0_101. Adv Exp Med Biol. 2016. PMID: 26427486 Free PMC article.
Cited by
-
Sensitivity of the Dorsal-Central Retinal Pigment Epithelium to Sodium Iodate-Induced Damage Is Associated With Overlying M-Cone Photoreceptors in Mice.Invest Ophthalmol Vis Sci. 2022 Aug 2;63(9):29. doi: 10.1167/iovs.63.9.29. Invest Ophthalmol Vis Sci. 2022. PMID: 36018572 Free PMC article.
-
Laminin-defined mechanical status modulates retinal pigment epithelium phagocytosis.EMBO Rep. 2025 Jul;26(13):3357-3383. doi: 10.1038/s44319-025-00475-9. Epub 2025 May 19. EMBO Rep. 2025. PMID: 40389756 Free PMC article.
-
Subretinal delivery of AAV5-mediated human Pde6b gene ameliorates the disease phenotype in a rat model of retinitis pigmentosa.Mol Vis. 2025 Mar 29;31:127-141. eCollection 2025. Mol Vis. 2025. PMID: 40384764 Free PMC article.
-
Single-Cell RNA Sequencing Reveals Molecular Features of Heterogeneity in the Murine Retinal Pigment Epithelium.Int J Mol Sci. 2022 Sep 8;23(18):10419. doi: 10.3390/ijms231810419. Int J Mol Sci. 2022. PMID: 36142331 Free PMC article.
-
SIRT6 overexpression in the nucleus protects mouse retinal pigment epithelium from oxidative stress.Life Sci Alliance. 2023 Apr 25;6(7):e202201448. doi: 10.26508/lsa.202201448. Print 2023 Jul. Life Sci Alliance. 2023. PMID: 37185874 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

