Underreporting of Symptomatic Adverse Events in Phase I Clinical Trials
- PMID: 33616650
- PMCID: PMC8502480
- DOI: 10.1093/jnci/djab015
Underreporting of Symptomatic Adverse Events in Phase I Clinical Trials
Abstract
Background: Clinician reporting of symptomatic adverse events (AEs) in phase I trials uses the Common Terminology Criteria for Adverse Events (CTCAE). The utility of the patient-reported outcomes (PROs) version of the CTCAE (PRO-CTCAE) in this setting is unknown. This prospective, observational study compared patient- and clinician-reported symptomatic AEs in phase I patients.
Methods: Phase I study-eligible patients at Princess Margaret were surveyed with the PRO-CTCAE full-item library (78 symptomatic AEs) at baseline (BL), mid-cycle 1, and mid-cycle 2 (C2). Patient and trial characteristics, best response, and survival data were collected. Presence or absence of patient- (PRO-CTCAE) or clinician-reported symptomatic AEs were compared (kappa) at defined timepoints and overall (BL+ mid-cycle 1 + C2).
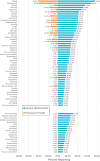
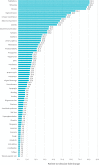
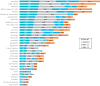
Results: Of 292 patients approached from May 2017 to January 2019, a total of 265 (90.8%) were consented, with 243 (91.7%) evaluable and 552 PRO-CTCAE surveys (completion rate = 98.7%) included in analyses. Evaluation of overall patient-reported symptomatic AEs identified 50 PRO-CTCAE and 11 CTCAE items with 10% or greater reporting frequency. Nineteen CTCAE items were reported as 1% or less despite matched PRO-CTCAE items reporting as 10% or greater. Underreported categories included sexual health, bodily emissions, and cognition. Clinician- relative to patient-reporting frequency (ratio) demonstrated 9 symptomatic AEs with a 50-fold or more lower clinician reporting rate. Overall patient-clinician agreement for individual symptomatic AEs ranged from poor (κ = 0.00-0.19) to moderate (κ = 0.40-0.59), with discordance driven by lack of clinician reporting. Dyspnea (κ = 0.54) and peripheral neuropathy (κ = 0.63) at BL and limb edema (κ = 0.55) at C2 demonstrated the highest patient-clinician agreement.
Conclusions: Poor to moderate patient-clinician agreement for symptomatic AEs suggests clinician underreporting in phase I trials. Analyses of severity and interference PRO categories are ongoing.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Assessment of Adverse Events From the Patient Perspective in a Phase 3 Metastatic Castration-Resistant Prostate Cancer Clinical Trial.JAMA Oncol. 2020 Feb 1;6(2):e193332. doi: 10.1001/jamaoncol.2019.3332. Epub 2020 Feb 13. JAMA Oncol. 2020. PMID: 31556911 Free PMC article. Clinical Trial.
-
Mapping child and adolescent self-reported symptom data to clinician-reported adverse event grading to improve pediatric oncology care and research.Cancer. 2020 Jan 1;126(1):140-147. doi: 10.1002/cncr.32525. Epub 2019 Sep 25. Cancer. 2020. PMID: 31553494 Free PMC article.
-
Patient free text reporting of symptomatic adverse events in cancer clinical research using the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).J Am Med Inform Assoc. 2019 Apr 1;26(4):276-285. doi: 10.1093/jamia/ocy169. J Am Med Inform Assoc. 2019. PMID: 30840079 Free PMC article.
-
Patient-Reported Outcomes in Cancer Clinical Trials: Measuring Symptomatic Adverse Events With the National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE).Am Soc Clin Oncol Educ Book. 2016;35:67-73. doi: 10.1200/EDBK_159514. Am Soc Clin Oncol Educ Book. 2016. PMID: 27249687 Review.
-
Clinician and Patient Reporting of Symptomatic Adverse Events in Cancer Clinical Trials: Using CTCAE and PRO-CTCAE® to Provide Two Distinct and Complementary Perspectives.Patient Relat Outcome Meas. 2022 Dec 8;13:249-258. doi: 10.2147/PROM.S256567. eCollection 2022. Patient Relat Outcome Meas. 2022. PMID: 36524232 Free PMC article. Review.
Cited by
-
Using Patient-Reported Outcomes in Dose-Finding Oncology Trials: Surveys of Key Stakeholders and the National Cancer Research Institute Consumer Forum.Oncologist. 2022 Sep 2;27(9):768-777. doi: 10.1093/oncolo/oyac117. Oncologist. 2022. PMID: 35762393 Free PMC article.
-
Statistical methods and data visualisation of patient-reported outcomes in early phase dose-finding oncology trials: a methodological review.EClinicalMedicine. 2023 Sep 21;64:102228. doi: 10.1016/j.eclinm.2023.102228. eCollection 2023 Oct. EClinicalMedicine. 2023. PMID: 37781154 Free PMC article.
-
Older adults with advanced cancer report pain not captured by clinician-graded Common Terminology Criteria for Adverse Events (CTCAE).J Geriatr Oncol. 2023 Apr;14(3):101480. doi: 10.1016/j.jgo.2023.101480. Epub 2023 Mar 28. J Geriatr Oncol. 2023. PMID: 36989940 Free PMC article. No abstract available.
-
Leveraging Patient-Reported Outcome Measures for Optimal Dose Selection in Early Phase Cancer Trials.JMIR Cancer. 2025 Feb 28;11:e64611. doi: 10.2196/64611. JMIR Cancer. 2025. PMID: 40020239 Free PMC article. Review.
-
Patient-centered dosing: oncologists' perspectives about treatment-related side effects and individualized dosing for patients with metastatic breast cancer (MBC).Breast Cancer Res Treat. 2022 Dec;196(3):549-563. doi: 10.1007/s10549-022-06755-5. Epub 2022 Oct 6. Breast Cancer Res Treat. 2022. PMID: 36198984
References
-
- Di Maio M, Basch E, Bryce J, Perrone F.. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. 2016;13(5):319–325. - PubMed
-
- Fromme EK, Eilers KM, Mori M, Hsieh Y-C, Beer TM.. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the quality-of-life questionnaire C30. J Clin Oncol. 2004;22(17):3485–3490. - PubMed
-
- Cirillo M, Venturini M, Ciccarelli L, Coati F, Bortolami O, Verlato G.. Clinician versus nurse symptom reporting using the National Cancer Institute—Common Terminology Criteria for Adverse Events during chemotherapy: results of a comparison based on patient’s self-reported questionnaire. Ann Oncol. 2009;20(12):1929–1935. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

