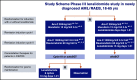
Addition of lenalidomide to intensive treatment in younger and middle-aged adults with newly diagnosed AML: the HOVON-SAKK-132 trial
- PMID: 33616652
- PMCID: PMC7903238
- DOI: 10.1182/bloodadvances.2020003855
Addition of lenalidomide to intensive treatment in younger and middle-aged adults with newly diagnosed AML: the HOVON-SAKK-132 trial
Abstract
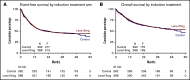
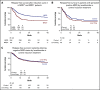
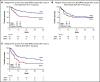
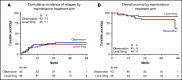
Lenalidomide, an antineoplastic and immunomodulatory drug, has therapeutic activity in acute myeloid leukemia (AML), but definitive studies about its therapeutic utility have been lacking. In a phase 3 study, we compared 2 induction regimens in newly diagnosed patients age 18 to 65 years with AML: idarubicine-cytarabine (cycle 1) and daunorubicin and intermediate-dose cytarabine (cycle 2) without or with lenalidomide (15 mg orally on days 1-21). One final consolidation cycle of chemotherapy or autologous stem cell transplantation (auto-SCT) or allogeneic SCT (allo-SCT) was provided according to a prognostic risk and minimal residual disease (MRD)-adapted approach. Event-free survival (EFS; primary end point) and other clinical end points were assessed. A second random assignment in patients in complete response or in complete response with incomplete hematologic recovery after cycle 3 or auto-SCT involved 6 cycles of maintenance with lenalidomide (10 mg on days 1-21) or observation. In all, 392 patients were randomly assigned to the control group, and 388 patients were randomly assigned to lenalidomide induction. At a median follow-up of 41 months, the study revealed no differences in outcome between the treatments (EFS, 44% ± 2% standard error and overall survival, 54% ± 2% at 4 years for both arms) although in an exploratory post hoc analysis, a lenalidomide benefit was suggested in SRSF2-mutant AML. In relation to the previous Dutch-Belgian Hemato-Oncology Cooperative Group and Swiss Group for Clinical Cancer Research (HOVON-SAKK) studies that used a similar 3-cycle regimen but did not pursue an MRD-guided approach, these survival estimates compare markedly more favorably. MRD status after cycle 2 lost prognostic value in intermediate-risk AML in the risk-adjusted treatment context. Maintenance with lenalidomide showed no apparent effect on relapse probability in 88 patients randomly assigned for this part of the study.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Löwenberg B, Pabst T, Maertens J, et al. ; Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) . Therapeutic value of clofarabine in younger and middle-aged (18-65 years) adults with newly diagnosed AML. Blood. 2017;129(12):1636-1645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

