Use of Mechanical Circulatory Support Devices Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock
- PMID: 33616664
- PMCID: PMC7900859
- DOI: 10.1001/jamanetworkopen.2020.37748
Use of Mechanical Circulatory Support Devices Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock
Abstract
Importance: Mechanical circulatory support (MCS) devices, including intravascular microaxial left ventricular assist devices (LVADs) and intra-aortic balloon pumps (IABPs), are used in patients who undergo percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI) complicated by cardiogenic shock despite limited evidence of their clinical benefit.
Objective: To examine trends in the use of MCS devices among patients who underwent PCI for AMI with cardiogenic shock, hospital-level use variation, and factors associated with use.
Design, setting, and participants: This cross-sectional study used the CathPCI and Chest Pain-MI Registries of the American College of Cardiology National Cardiovascular Data Registry. Patients who underwent PCI for AMI complicated by cardiogenic shock between October 1, 2015, and December 31, 2017, were identified from both registries. Data were analyzed from October 2018 to August 2020.
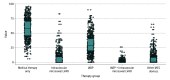
Exposures: Therapies to provide hemodynamic support were categorized as intravascular microaxial LVAD, IABP, TandemHeart, extracorporeal membrane oxygenation, LVAD, other devices, combined IABP and intravascular microaxial LVAD, combined IABP and other device (defined as TandemHeart, extracorporeal membrane oxygenation, LVAD, or another MCS device), or medical therapy only.
Main outcomes and measures: Use of MCS devices overall and specific MCS devices, including intravascular microaxial LVAD, at both patient and hospital levels and variables associated with use.
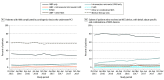
Results: Among the 28 304 patients included in the study, the mean (SD) age was 65.4 (12.6) years and 18 968 were men (67.0%). The overall MCS device use was constant from the fourth quarter of 2015 to the fourth quarter of 2017, although use of intravascular microaxial LVADs significantly increased (from 4.1% to 9.8%; P < .001), whereas use of IABPs significantly decreased (from 34.8% to 30.0%; P < .001). A significant hospital-level variation in MCS device use was found. The median (interquartile range [IQR]) proportion of patients who received MCS devices was 42% (30%-54%), and the median proportion of patients who received intravascular microaxial LVADs was 1% (0%-10%). In multivariable analyses, cardiac arrest at first medical contact or during hospitalization (odds ratio [OR], 1.82; 95% CI, 1.58-2.09) and severe left main and/or proximal left anterior descending coronary artery stenosis (OR, 1.36; 95% CI, 1.20-1.54) were patient characteristics that were associated with higher odds of receiving intravascular microaxial LVADs only compared with IABPs only.
Conclusions and relevance: This study found that, among patients who underwent PCI for AMI complicated by cardiogenic shock, overall use of MCS devices was constant, and a 2.5-fold increase in intravascular microaxial LVAD use was found along with a corresponding decrease in IABP use and a significant hospital-level variation in MCS device use. These trends were observed despite limited clinical trial evidence of improved outcomes associated with device use.
Conflict of interest statement
Figures
References
-
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):e78-e140. doi: 10.1016/j.jacc.2012.11.019 - DOI - PubMed
-
- Thiele H, Zeymer U, Neumann FJ, et al. ; Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) Trial Investigators . Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382(9905):1638-1645. doi: 10.1016/S0140-6736(13)61783-3 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

