First-in-human study demonstrating the safety and clinical efficacy of novel anti-IL-17A monoclonal antibody CJM112 in moderate to severe plaque psoriasis
- PMID: 33617042
- PMCID: PMC8246720
- DOI: 10.1111/jdv.17071
First-in-human study demonstrating the safety and clinical efficacy of novel anti-IL-17A monoclonal antibody CJM112 in moderate to severe plaque psoriasis
Abstract
Background and objective: Anti-IL-17A IgG/κ monoclonal antibody CJM112 binds both IL-17A and IL-17AF. The purpose of this First-in-Human study was to assess CJM112 effects on safety and efficacy in patients with moderate to severe plaque psoriasis.
Methods: This study had two parts: single ascending doses of 5-450 mg subcutaneous (s.c.) CJM112 (SAD) and multi-dose parallel groups of CJM112 15 mg, 50 mg and 150 mg s.c. low frequency or high frequency (MD). SAD/MD were double-blind, randomized and placebo-controlled; MD also included a secukinumab 150 mg s.c. arm as an active comparator. Patients 18-65 years with moderate to severe psoriasis were included in this study. The efficacy outcome was the change in Psoriasis Area Severity Index (PASI) from baseline to Week 4 in the SAD part of the study, and from baseline to Week 12 in the MD part.
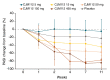
Results: 96 patients were enrolled in this study (SAD, n = 42; MD, n = 54). In SAD, CJM112 doses from 15 mg and above demonstrated higher PASI responses compared with placebo at Week 12. CJM112 450 mg did not add further efficacy, but efficacy duration was prolonged compared with CJM112 150 mg. CJM112 MD resulted in a dose-dependent decrease in PASI over time to Week 12. CJM112 150 mg high frequency did not exceed the effect of CJM112 150 mg low frequency and had similar efficacy to secukinumab 150 mg. The safety profile of CJM112 was as expected for an antibody targeting IL-17A/IL-17AF.
Conclusions: CJM112 had clinical efficacy in moderate to severe psoriasis and was generally safe and well tolerated in the doses tested. Additional neutralization of IL-17AF did not translate to increased clinical efficacy compared with secukinumab.
© 2020 Novartis Pharma AG. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures


References
-
- Gupta MA, Gupta AK. Depression and suicidal ideation in dermatology patients with acne, alopecia areata, atopic dermatitis and psoriasis. Br J Dermatol 1998; 139: 846–850. - PubMed
-
- Christophers E, Barker JN, Griffiths CE, Dauden E, Milligan G, Molta C et al. The risk of psoriatic arthritis remains constant following initial diagnosis of psoriasis among patients seen in European dermatology clinics. J Eur Acad Dermatol Venereol 2010; 24: 548–554. - PubMed
-
- Gisondi P, Fostini AC, Fossa I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clin Dermatol 2018; 36: 21–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

