TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men With Castration-Resistant Metastatic Prostate Cancer
- PMID: 33617303
- PMCID: PMC8274807
- DOI: 10.1200/JCO.20.02759
TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men With Castration-Resistant Metastatic Prostate Cancer
Abstract
Purpose: Prostate cancer (PCa) becomes resistant to androgen ablation through adaptive upregulation of the androgen receptor in response to the low-testosterone microenvironment. Bipolar androgen therapy (BAT), defined as rapid cycling between high and low serum testosterone, disrupts this adaptive regulation in castration-resistant PCa (CRPC).
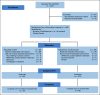
Methods: The TRANSFORMER (Testosterone Revival Abolishes Negative Symptoms, Fosters Objective Response and Modulates Enzalutamide Resistance) study is a randomized study comparing monthly BAT (n = 94) with enzalutamide (n = 101). The primary end point was clinical or radiographic progression-free survival (PFS); crossover was permitted at progression. Secondary end points included overall survival (OS), prostate-specific antigen (PSA) and objective response rates, PFS from randomization through crossover (PFS2), safety, and quality of life (QoL).
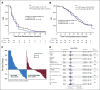
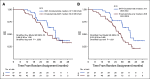
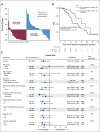
Results: The PFS was 5.7 months for both arms (hazard ratio [HR], 1.14; 95% CI, 0.83 to 1.55; P = .42). For BAT, 50% decline in PSA (PSA50) was 28.2% of patients versus 25.3% for enzalutamide. At crossover, PSA50 response occurred in 77.8% of patients crossing to enzalutamide and 23.4% to BAT. The PSA-PFS for enzalutamide increased from 3.8 months after abiraterone to 10.9 months after BAT. The PFS2 for BAT→enzalutamide was 28.2 versus 19.6 months for enzalutamide→BAT (HR, 0.44; 95% CI, 0.22 to 0.88; P = .02). OS was 32.9 months for BAT versus 29.0 months for enzalutamide (HR, 0.95; 95% CI, 0.66 to 1.39; P = .80). OS was 37.1 months for patients crossing from BAT to enzalutamide versus 30.2 months for the opposite sequence (HR, 0.68; 95% CI, 0.36 to 1.28; P = .225). BAT adverse events were primarily grade 1-2. Patient-reported QoL consistently favored BAT.
Conclusion: This randomized trial establishes meaningful clinical activity and safety of BAT and supports additional study to determine its optimal clinical integration. BAT can sensitize CRPC to subsequent antiandrogen therapy. Further study is required to confirm whether sequential therapy with BAT and enzalutamide can improve survival in men with CRPC.
Trial registration: ClinicalTrials.gov NCT02286921.
Conflict of interest statement
Figures
References
-
- Huggins C, Hodges CV.Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate Cancer Res 1293–2971941 - PubMed
-
- Emamekhoo H, Barata PC, Edwin NC, et al. Evaluation of response to enzalutamide consecutively after abiraterone acetate/prednisone failure in patients with metastatic castration-resistant PCa Clin Genitourin Cancer 16429–4362018 - PubMed
-
- Smith MR, Saad F, Rathkopf DE, et al. Clinical outcomes from androgen signaling-directed therapy after treatment with abiraterone acetate and prednisone in patients with metastatic castration-resistant PCa: Post hoc analysis of COU-AA-302 Eur Urol 7210–132017 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

