The role of complement in brain injury following intracerebral hemorrhage: A review
- PMID: 33617886
- PMCID: PMC8119338
- DOI: 10.1016/j.expneurol.2021.113654
The role of complement in brain injury following intracerebral hemorrhage: A review
Abstract
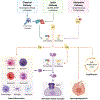
Intracerebral hemorrhage (ICH) is a significant cause of death and disability and current treatment is limited to supportive measures to reduce brain edema and secondary hematoma expansion. Current evidence suggests that the complement cascade is activated early after hemorrhage and contributes to brain edema/injury in multiple ways. The aim of this review is to summarize the most recent literature about the role of the complement cascade after ICH. Primary literature demonstrating complement mediated brain edema and neurologic injury through the membrane attack complex (MAC) as well as C3a and C5a are reviewed. Further, attenuation of brain edema and improved functional outcomes are demonstrated after inhibition of specific components of the complement cascade. Conversely, complement also plays a significant role in neurologic recovery after ICH and in other neurologic disorders. We conclude that the role of complement after ICH is complex. Understanding the role of complement after ICH is essential and may elucidate possible interventions to reduce brain edema and injury.
Keywords: Brain injury; Complement; Intervention; Intracerebral hemorrhage.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
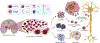

Similar articles
-
Revisiting the role of the complement system in intracerebral hemorrhage and therapeutic prospects.Int Immunopharmacol. 2023 Oct;123:110744. doi: 10.1016/j.intimp.2023.110744. Epub 2023 Aug 7. Int Immunopharmacol. 2023. PMID: 37552908
-
Complement activation in the brain after experimental intracerebral hemorrhage.J Neurosurg. 2000 Jun;92(6):1016-22. doi: 10.3171/jns.2000.92.6.1016. J Neurosurg. 2000. PMID: 10839264
-
Role of complement C1q/C3-CR3 signaling in brain injury after experimental intracerebral hemorrhage and the effect of minocycline treatment.Front Immunol. 2022 Sep 15;13:919444. doi: 10.3389/fimmu.2022.919444. eCollection 2022. Front Immunol. 2022. PMID: 36189326 Free PMC article.
-
Role of flunarizine hydrochloride in secondary brain injury following intracerebral hemorrhage in rats.Int J Immunopathol Pharmacol. 2017 Dec;30(4):413-419. doi: 10.1177/0394632017742224. Epub 2017 Nov 22. Int J Immunopathol Pharmacol. 2017. Retraction in: Int J Immunopathol Pharmacol. 2021 Jan-Dec;35:20587384211040393. doi: 10.1177/20587384211040393. PMID: 29164980 Free PMC article. Retracted.
-
The complement cascade as a therapeutic target in intracerebral hemorrhage.Exp Neurol. 2009 Oct;219(2):398-403. doi: 10.1016/j.expneurol.2009.07.018. Epub 2009 Jul 24. Exp Neurol. 2009. PMID: 19632224 Free PMC article. Review.
Cited by
-
Molecular, Pathological, Clinical, and Therapeutic Aspects of Perihematomal Edema in Different Stages of Intracerebral Hemorrhage.Oxid Med Cell Longev. 2022 Sep 17;2022:3948921. doi: 10.1155/2022/3948921. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36164392 Free PMC article. Review.
-
"Rogue" [DEspR+CD11b+] neutrophil subset correlates with severity in spontaneous intracerebral hemorrhage.Front Neurol. 2022 Jul 25;13:935579. doi: 10.3389/fneur.2022.935579. eCollection 2022. Front Neurol. 2022. PMID: 35959408 Free PMC article.
-
Mechanisms of hydrocephalus after intraventricular haemorrhage: a review.Childs Nerv Syst. 2024 Dec 15;41(1):49. doi: 10.1007/s00381-024-06711-2. Childs Nerv Syst. 2024. PMID: 39674974 Review.
-
A new perspective on the regulation of neuroinflammation in intracerebral hemorrhage: mechanisms of NLRP3 inflammasome activation and therapeutic strategies.Front Immunol. 2025 Feb 27;16:1526786. doi: 10.3389/fimmu.2025.1526786. eCollection 2025. Front Immunol. 2025. PMID: 40083546 Free PMC article. Review.
-
The Role of Complement C1qa in Experimental Intracerebral Hemorrhage.Transl Stroke Res. 2025 Aug;16(4):1229-1240. doi: 10.1007/s12975-024-01302-4. Epub 2024 Oct 7. Transl Stroke Res. 2025. PMID: 39370487
References
-
- Appelboom G, Piazza M, Hwang BY, Bruce S, Smith S, Bratt A, Bagiella E, Badjatia N, Mayer S, Sander Connolly E, 2011. Complement Factor H Y402H polymorphism is associated with an increased risk of mortality after intracerebral hemorrhage. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 18, 1439–1443. - PubMed
-
- Aronowski J, Hall CE, 2005. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurological research 27, 268–279. - PubMed
-
- Bellander BM, Singhrao SK, Ohlsson M, Mattsson P, Svensson M, 2001. Complement activation in the human brain after traumatic head injury. Journal of neurotrauma 18, 1295–1311. - PubMed
-
- Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, Mayberg M, Morgenstern L, Ogilvy CS, Vespa P, Zuccarello M, American Heart A, American Stroke Association Stroke, C., High Blood Pressure Research, C., Quality of, C., Outcomes in Research Interdisciplinary Working, G., 2007. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke 38, 2001–2023. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

