Patient-Derived Mutant Forms of NFE2L2/NRF2 Drive Aggressive Murine Hepatoblastomas
- PMID: 33618031
- PMCID: PMC8102178
- DOI: 10.1016/j.jcmgh.2021.02.004
Patient-Derived Mutant Forms of NFE2L2/NRF2 Drive Aggressive Murine Hepatoblastomas
Abstract
Background & aims: Hepatoblastoma (HB), the most common pediatric liver cancer, often bears β-catenin mutations and deregulates the Hippo tumor suppressor pathway. Murine HBs can be generated by co-expressing β-catenin mutants and the constitutively active Hippo effector YAPS127A. Some HBs and other cancers also express mutants of NFE2L2/NRF2 (NFE2L2), a transcription factor that tempers oxidative and electrophilic stress. In doing so, NFE2L2 either suppresses or facilitates tumorigenesis.
Methods: We evaluated NFE2L2's role in HB pathogenesis by co-expressing all combinations of mutant β-catenin, YAPS127A, and the patient-derived NFE2L2 mutants L30P and R34P in murine livers. We evaluated growth, biochemical and metabolic profiles, and transcriptomes of the ensuing tumors.
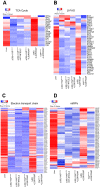
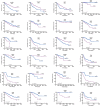
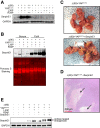
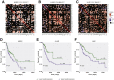
Results: In association with β-catenin+YAPS127A, L30P and R34P markedly accelerated HB growth and generated widespread cyst formation and necrosis, which are otherwise uncommon features. Surprisingly, any 2 members of the mutant β-catenin-YAPS127A-L30P/R34P triad were tumorigenic, thus directly establishing NFE2L2's oncogenicity. Each tumor group displayed distinct features but shared 22 similarly deregulated transcripts, 10 of which perfectly correlated with survival in human HBs and 17 of which correlated with survival in multiple adult cancers. One highly up-regulated transcript encoded serpin E1, a serine protease inhibitor that regulates fibrinolysis, growth, and extracellular matrix. Although the combination of mutant β-catenin, YAPS127A, and serpin E1 did not accelerate cystogenic tumor growth, it did promote the widespread necrosis associated with mutant β-catenin-YAPS127A-L30P/R34P tumors.
Conclusions: Our findings establish the direct oncogenicity of NFE2L2 mutants and key transcripts, including serpin E1, that drive specific HB features.
Keywords: Hepatocellular Carcinoma; KEAP1; Plasminogen Activator Inhibitor; Warburg Effect.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures









Comment in
-
Help for Sick Kids: New Insights Into Hepatoblastoma.Cell Mol Gastroenterol Hepatol. 2021;12(1):350-351. doi: 10.1016/j.jcmgh.2021.03.001. Epub 2021 Mar 26. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33775655 Free PMC article. No abstract available.
References
-
- Czauderna P., Lopez-Terrada D., Hiyama E., Haberle B., Malogolowkin M.H., Meyers R.L. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr. 2014;26:19–28. - PubMed
-
- Tao J., Calvisi D.F., Ranganathan S., Cigliano A., Zhou L., Singh S., Jiang L., Fan B., Terracciano L., Armeanu-Ebinger S., Ribback S., Dombrowski F., Evert M., Chen X., Monga S.P.S. Activation of beta-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology. 2014;147:690–701. - PMC - PubMed
-
- Grobner S.N., Worst B.C., Weischenfeldt J., Buchhalter I., Kleinheinz K., Rudneva V.A., Johann P.D., Balasubramanian G.P., Segura-Wang M., Brabetz S., Bender S., Hutter B., Sturm D., Pfaff E., Hubschmann D., Zipprich G., Heinold M., Eils J., Lawerenz C., Erkek S., Lambo S., Waszak S., Blattmann C., Borkhardt A., Kuhlen M., Eggert A., Fulda S., Gessler M., Wegert J., Kappler R., Baumhoer D., Burdach S., Kirschner-Schwabe R., Kontny U., Kulozik A.E., Lohmann D., Hettmer S., Eckert C., Bielack S., Nathrath M., Niemeyer C., Richter G.H., Schulte J., Siebert R., Westermann F., Molenaar J.J., Vassal G., Witt H., IP-S Project, IM-S Project, Burkhardt B., Kratz C.P., Witt O., van Tilburg C.M., Kramm C.M., Fleischhack G., Dirksen U., Rutkowski S., Fruhwald M., von Hoff K., Wolf S., Klingebiel T., Koscielniak E., Landgraf P, Koster J., Resnick A.C., Zhang J., Liu Y., Zhou X., Waanders A.J., Zwijnenburg D.A., Raman P., Brors B., Weber U.D., Northcott P.A., Pajtler K.W., Kool M., Piro R.M., Korbel J.O., Schlesner M., Eils R., Jones D.T.W., Lichter P., Chavez L., Zapatka M., Pfister S.M. The landscape of genomic alterations across childhood cancers. Nature. 2018;555:321–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

