Faropenem reacts with serine and metallo-β-lactamases to give multiple products
- PMID: 33618159
- PMCID: PMC7614720
- DOI: 10.1016/j.ejmech.2021.113257
Faropenem reacts with serine and metallo-β-lactamases to give multiple products
Abstract
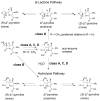
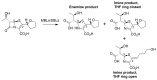
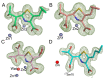
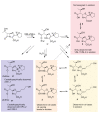
Penems have demonstrated potential as antibacterials and β-lactamase inhibitors; however, their clinical use has been limited, especially in comparison with the structurally related carbapenems. Faropenem is an orally active antibiotic with a C-2 tetrahydrofuran (THF) ring, which is resistant to hydrolysis by some β-lactamases. We report studies on the reactions of faropenem with carbapenem-hydrolysing β-lactamases, focusing on the class A serine β-lactamase KPC-2 and the metallo β-lactamases (MBLs) VIM-2 (a subclass B1 MBL) and L1 (a B3 MBL). Kinetic studies show that faropenem is a substrate for all three β-lactamases, though it is less efficiently hydrolysed by KPC-2. Crystallographic analyses on faropenem-derived complexes reveal opening of the β-lactam ring with formation of an imine with KPC-2, VIM-2, and L1. In the cases of the KPC-2 and VIM-2 structures, the THF ring is opened to give an alkene, but with L1 the THF ring remains intact. Solution state studies, employing NMR, were performed on L1, KPC-2, VIM-2, VIM-1, NDM-1, OXA-23, OXA-10, and OXA-48. The solution results reveal, in all cases, formation of imine products in which the THF ring is opened; formation of a THF ring-closed imine product was only observed with VIM-1 and VIM-2. An enamine product with a closed THF ring was also observed in all cases, at varying levels. Combined with previous reports, the results exemplify the potential for different outcomes in the reactions of penems with MBLs and SBLs and imply further structure-activity relationship studies are worthwhile to optimise the interactions of penems with β-lactamases. They also exemplify how crystal structures of β-lactamase substrate/inhibitor complexes do not always reflect reaction outcomes in solution.
Keywords: Antimicrobial resistance; Carbapenems; Metallo-β-lactamases; Penems; Serine-β-lactamases; β-Lactams.
Copyright © 2021. Published by Elsevier Masson SAS.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Bush K. Metallo-beta-lactamases: a class apart. [accessed May 29, 2018];Clin Infect Dis. 1998 27(Suppl 1):S48–53. - PubMed
-
- Lisa M-N, Palacios AR, Aitha M, González MM, Moreno DM, Crowder MW, Bonomo RA, Spencer J, Tierney DL, Llarrull LI, Vila AJ. A general reaction mechanism for carbapenem hydrolysis by mononuclear and binuclear metallo-β-lactamases. Nat Commun. 2017;8:538. doi: 10.1038/s41467-017-00601-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

