Functional analysis of POLD1 p.ser605del variant: the aging phenotype of MDPL syndrome is associated with an impaired DNA repair capacity
- PMID: 33618333
- PMCID: PMC7950258
- DOI: 10.18632/aging.202680
Functional analysis of POLD1 p.ser605del variant: the aging phenotype of MDPL syndrome is associated with an impaired DNA repair capacity
Abstract
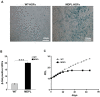
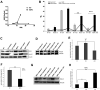
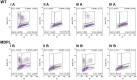
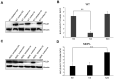
Mandibular hypoplasia, Deafness and Progeroid features with concomitant Lipodystrophy define a rare systemic disorder, named MDPL Syndrome, due to almost always a de novo variant in POLD1 gene, encoding the DNA polymerase δ. We report a MDPL female heterozygote for the recurrent p.Ser605del variant. In order to deepen the functional role of the in frame deletion affecting the polymerase catalytic site of the protein, cellular phenotype has been characterised. MDPL fibroblasts exhibit in vitro nuclear envelope anomalies, accumulation of prelamin A and presence of micronuclei. A decline of cell growth, cellular senescence and a blockage of proliferation in G0/G1 phase complete the aged cellular picture. The evaluation of the genomic instability reveals a delayed recovery from DNA induced-damage. Moreover, the rate of telomere shortening was greater in pathological cells, suggesting the telomere dysfunction as an emerging key feature in MDPL. Our results suggest an alteration in DNA replication/repair function of POLD1 as a primary pathogenetic cause of MDPL. The understanding of the mechanisms linking these cellular characteristics to the accelerated aging and to the wide spectrum of affected tissues and clinical symptoms in the MDPL patients may provide opportunities to develop therapeutic treatments for progeroid syndromes.
Keywords: DNA repair; MDPL syndrome; POLD1 gene; age-related disease; telomere damage.
Conflict of interest statement
The authors want to dedicate the paper to a progeroid patient who died last month.
This paper is dedicated to the memory of Walter Palone who, with his voluntary availability as a patient, has allowed us to open a path on genetic premature aging and laminopathies.
Figures

References
-
- Shastry S, Simha V, Godbole K, Sbraccia P, Melancon S, Yajnik CS, Novelli G, Kroiss M, Garg A. A novel syndrome of mandibular hypoplasia, deafness, and progeroid features associated with lipodystrophy, undescended testes, and male hypogonadism. J Clin Endocrinol Metab. 2010; 95:E192–97. 10.1210/jc.2010-0419 - DOI - PMC - PubMed
-
- Ajluni N, Meral R, Neidert AH, Brady GF, Buras E, McKenna B, DiPaola F, Chenevert TL, Horowitz JF, Buggs-Saxton C, Rupani AR, Thomas PE, Tayeh MK, et al.. Spectrum of disease associated with partial lipodystrophy: lessons from a trial cohort. Clin Endocrinol (Oxf). 2017; 86:698–707. 10.1111/cen.13311 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

