Rational selection of a biomarker panel targeting unmet clinical needs in kidney injury
- PMID: 33618665
- PMCID: PMC7898424
- DOI: 10.1186/s12014-021-09315-z
Rational selection of a biomarker panel targeting unmet clinical needs in kidney injury
Abstract
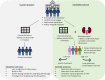
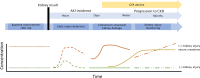
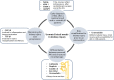
The pipeline of biomarker translation from bench to bedside is challenging and limited biomarkers have been adopted to routine clinical care. Ideally, biomarker research and development should be driven by unmet clinical needs in health care. To guide researchers, clinical chemists and clinicians in their biomarker research, the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) has developed a structured questionnaire in which the clinical gaps in current clinical pathways are identified and desirable performance specifications are predefined. In kidney injury, the high prevalence of the syndrome acute kidney injury (AKI) in the hospital setting has a significant impact on morbidity, patient survival and health care costs, but the use of biomarkers indicating early kidney injury in daily patient care remains limited. Routinely, medical labs measure serum creatinine, which is a functional biomarker, insensitive for detecting early kidney damage and cannot distinguish between renal and prerenal AKI. The perceived unmet clinical needs in kidney injury were identified through the EFLM questionnaire. Nephrologists within our tertiary care hospital emphasized that biomarkers are needed for (1) early diagnosis of in-hospital AKI after a medical insult and in critically ill patients, (2) risk stratification for kidney injury prior to a scheduled (elective) intervention, (3) kidney injury monitoring in patients scheduled to receive nephrotoxic medication and after kidney transplantation and (4) differentiation between prerenal AKI and structural kidney damage. The biomarker search and selection strategy resulted in a rational selection of an eleven-protein urinary panel for kidney injury that target these clinical needs. To assess the clinical utility of the proposed biomarker panel in kidney injury, a multiplexed LC-MS test is now in development for the intended translational research.
Keywords: Acute kidney injury; Biomarkers; Clinical needs; Urinalysis; Urine proteomics.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Horvath AR, Lord SJ, StJohn A, Sandberg S, Cobbaert CM, Lorenz S, Monaghan PJ, Verhagen-Kamerbeek WD, Ebert C, Bossuyt PM, et al. From biomarkers to medical tests: the changing landscape of test evaluation. Clin Chim Acta Int J Clin Chem. 2014;427:49–57. doi: 10.1016/j.cca.2013.09.018. - DOI - PubMed
-
- Lord SJ, St John A, Bossuyt PM, Sandberg S, Monaghan PJ, O'Kane M, Cobbaert CM, Roddiger R, Lennartz L, Gelfi C, et al. Setting clinical performance specifications to develop and evaluate biomarkers for clinical use. Ann Clin Biochem. 2019;56(5):527–535. doi: 10.1177/0004563219842265. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
