Developing synergistic drug combinations to restore antibiotic sensitivity in drug-resistant Mycobacterium tuberculosis
- PMID: 33619062
- PMCID: PMC8092878
- DOI: 10.1128/AAC.02554-20
Developing synergistic drug combinations to restore antibiotic sensitivity in drug-resistant Mycobacterium tuberculosis
Abstract
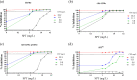
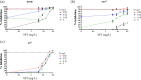
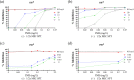
Tuberculosis (TB) is a leading global cause of mortality owing to an infectious agent, accounting for almost one-third of antimicrobial resistance (AMR) deaths annually. We aimed to identify synergistic anti-TB drug combinations with the capacity to restore therapeutic efficacy against drug-resistant mutants of the causative agent, Mycobacterium tuberculosis We investigated combinations containing the known translational inhibitors, spectinomycin (SPT) and fusidic acid (FA), or the phenothiazine, chlorpromazine (CPZ), which disrupts mycobacterial energy metabolism. Potentiation of whole-cell drug efficacy was observed in SPT-CPZ combinations. This effect was lost against an M. tuberculosis mutant lacking the major facilitator superfamily (MFS) efflux pump, Rv1258c. Notably, the SPT-CPZ combination partially restored SPT efficacy against an SPT-resistant mutant carrying a g1379t point mutation in rrs, encoding the mycobacterial 16S ribosomal RNA. Combinations of SPT with FA, which targets the mycobacterial elongation factor G, exhibited potentiating activity against wild-type M. tuberculosis Moreover, this combination produced a modest potentiating effect against both FA-monoresistant and SPT-monoresistant mutants. Finally, combining SPT with the frontline anti-TB agents, rifampicin (RIF) and isoniazid, resulted in enhanced activity in vitro and ex vivo against both drug-susceptible M. tuberculosis and a RIF-monoresistant rpoB S531L mutant.These results support the utility of novel potentiating drug combinations in restoring antibiotic susceptibility of M. tuberculosis strains carrying genetic resistance to any one of the partner compounds.
Copyright © 2021 Omollo et al.
Figures
Similar articles
-
Study of efflux pump gene expression in rifampicin-monoresistant Mycobacterium tuberculosis clinical isolates.J Antibiot (Tokyo). 2015 Jul;68(7):431-5. doi: 10.1038/ja.2015.9. Epub 2015 Feb 18. J Antibiot (Tokyo). 2015. PMID: 25690361
-
[Investigation of Efflux Pump Genes in Resistant Mycobacterium tuberculosis Complex Clinical Isolates Exposed to First Line Antituberculosis Drugs and Verapamil Combination].Mikrobiyol Bul. 2023 Apr;57(2):207-219. doi: 10.5578/mb.20239916. Mikrobiyol Bul. 2023. PMID: 37067206 Turkish.
-
Drug resistance pattern and mutation pattern in pediatric tuberculosis: Study from north India.Indian J Tuberc. 2021 Oct;68(4):481-484. doi: 10.1016/j.ijtb.2021.02.014. Epub 2021 Mar 2. Indian J Tuberc. 2021. PMID: 34752317
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
Efflux pumps in Mycobacterium tuberculosis and their inhibition to tackle antimicrobial resistance.Trends Microbiol. 2022 Jan;30(1):57-68. doi: 10.1016/j.tim.2021.05.001. Epub 2021 May 26. Trends Microbiol. 2022. PMID: 34052094 Review.
Cited by
-
In vitro model to simulate multiple drugs with distinct elimination half-lives.Int J Antimicrob Agents. 2023 Oct;62(4):106924. doi: 10.1016/j.ijantimicag.2023.106924. Epub 2023 Jul 9. Int J Antimicrob Agents. 2023. PMID: 37433386 Free PMC article.
-
Fixed-Dose Combination Formulations in Solid Oral Drug Therapy: Advantages, Limitations, and Design Features.Pharmaceutics. 2024 Jan 26;16(2):178. doi: 10.3390/pharmaceutics16020178. Pharmaceutics. 2024. PMID: 38399239 Free PMC article. Review.
-
The human proton pump inhibitors inhibit Mycobacterium tuberculosis rifampicin efflux and macrophage-induced rifampicin tolerance.Proc Natl Acad Sci U S A. 2023 Feb 14;120(7):e2215512120. doi: 10.1073/pnas.2215512120. Epub 2023 Feb 10. Proc Natl Acad Sci U S A. 2023. PMID: 36763530 Free PMC article.
-
Antimycobacterial Activity, Synergism, and Mechanism of Action Evaluation of Novel Polycyclic Amines against Mycobacterium tuberculosis.Adv Pharmacol Pharm Sci. 2021 Jun 11;2021:5583342. doi: 10.1155/2021/5583342. eCollection 2021. Adv Pharmacol Pharm Sci. 2021. PMID: 34240057 Free PMC article.
-
Ethambutol and meropenem/clavulanate synergy promotes enhanced extracellular and intracellular killing of Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2024 Apr 3;68(4):e0158623. doi: 10.1128/aac.01586-23. Epub 2024 Feb 27. Antimicrob Agents Chemother. 2024. PMID: 38411952 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

