Cross-reactivity of a pathogenic autoantibody to a tumor antigen in GABAA receptor encephalitis
- PMID: 33619082
- PMCID: PMC7936355
- DOI: 10.1073/pnas.1916337118
Cross-reactivity of a pathogenic autoantibody to a tumor antigen in GABAA receptor encephalitis
Abstract
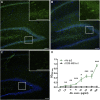
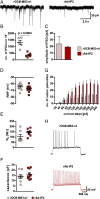
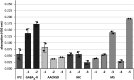
Encephalitis associated with antibodies against the neuronal gamma-aminobutyric acid A receptor (GABAA-R) is a rare form of autoimmune encephalitis. The pathogenesis is still unknown but autoimmune mechanisms were surmised. Here we identified a strongly expanded B cell clone in the cerebrospinal fluid of a patient with GABAA-R encephalitis. We expressed the antibody produced by it and showed by enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry that it recognizes the GABAA-R. Patch-clamp recordings revealed that it tones down inhibitory synaptic transmission and causes increased excitability of hippocampal CA1 pyramidal neurons. Thus, the antibody likely contributed to clinical disease symptoms. Hybridization to a protein array revealed the cross-reactive protein LIM-domain-only protein 5 (LMO5), which is related to cell-cycle regulation and tumor growth. We confirmed LMO5 recognition by immunoprecipitation and ELISA and showed that cerebrospinal fluid samples from two other patients with GABAA-R encephalitis also recognized LMO5. This suggests that cross-reactivity between GABAA-R and LMO5 is frequent in GABAA-R encephalitis and supports the hypothesis of a paraneoplastic etiology.
Keywords: GABA-A-receptor encephalitis; autoantibody; autoimmune encephalitis; epilepsy; paraneoplastic encephalitis.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Dalmau J., Graus F., Antibody-mediated encephalitis. N. Engl. J. Med. 378, 840–851 (2018). - PubMed
-
- Vincent A., Bien C. G., Irani S. R., Waters P., Autoantibodies associated with diseases of the CNS: New developments and future challenges. Lancet Neurol. 10, 759–772 (2011). - PubMed
-
- van Coevorden-Hameete M. H., de Graaff E., Titulaer M. J., Hoogenraad C. C., Sillevis Smitt P. A., Molecular and cellular mechanisms underlying anti-neuronal antibody mediated disorders of the central nervous system. Autoimmun. Rev. 13, 299–312 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

