Encoding memory in tube diameter hierarchy of living flow network
- PMID: 33619174
- PMCID: PMC7958412
- DOI: 10.1073/pnas.2007815118
Encoding memory in tube diameter hierarchy of living flow network
Abstract
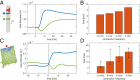
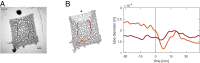
The concept of memory is traditionally associated with organisms possessing a nervous system. However, even very simple organisms store information about past experiences to thrive in a complex environment-successfully exploiting nutrient sources, avoiding danger, and warding off predators. How can simple organisms encode information about their environment? We here follow how the giant unicellular slime mold Physarum polycephalum responds to a nutrient source. We find that the network-like body plan of the organism itself serves to encode the location of a nutrient source. The organism entirely consists of interlaced tubes of varying diameters. Now, we observe that these tubes grow and shrink in diameter in response to a nutrient source, thereby imprinting the nutrient's location in the tube diameter hierarchy. Combining theoretical model and experimental data, we reveal how memory is encoded: a nutrient source locally releases a softening agent that gets transported by the cytoplasmic flows within the tubular network. Tubes receiving a lot of softening agent grow in diameter at the expense of other tubes shrinking. Thereby, the tubes' capacities for flow-based transport get permanently upgraded toward the nutrient location, redirecting future decisions and migration. This demonstrates that nutrient location is stored in and retrieved from the networks' tube diameter hierarchy. Our findings explain how network-forming organisms like slime molds and fungi thrive in complex environments. We here identify a flow networks' version of associative memory-very likely of relevance for the plethora of living flow networks as well as for bioinspired design.
Keywords: adaptive networks; behavior; decision making; flow networks.
Conflict of interest statement
The authors declare no competing interest.
Figures



Comment in
-
A slime mold's remembrance of things past.Proc Natl Acad Sci U S A. 2021 Apr 6;118(14):e2102056118. doi: 10.1073/pnas.2102056118. Proc Natl Acad Sci U S A. 2021. PMID: 33737448 Free PMC article. No abstract available.
-
Missing evidence for memory in the monocellular slime mold.Proc Natl Acad Sci U S A. 2021 Sep 7;118(36):e2105928118. doi: 10.1073/pnas.2105928118. Proc Natl Acad Sci U S A. 2021. PMID: 34470820 Free PMC article. No abstract available.
Similar articles
-
Mechanism of signal propagation in Physarum polycephalum.Proc Natl Acad Sci U S A. 2017 May 16;114(20):5136-5141. doi: 10.1073/pnas.1618114114. Epub 2017 May 2. Proc Natl Acad Sci U S A. 2017. PMID: 28465441 Free PMC article.
-
Random network peristalsis in Physarum polycephalum organizes fluid flows across an individual.Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):13306-11. doi: 10.1073/pnas.1305049110. Epub 2013 Jul 29. Proc Natl Acad Sci U S A. 2013. PMID: 23898203 Free PMC article.
-
A revised model of fluid transport optimization in Physarum polycephalum.J Math Biol. 2017 Feb;74(3):567-581. doi: 10.1007/s00285-016-1036-y. Epub 2016 Jun 11. J Math Biol. 2017. PMID: 27289474
-
Fluid flows shaping organism morphology.Philos Trans R Soc Lond B Biol Sci. 2018 May 26;373(1747):20170112. doi: 10.1098/rstb.2017.0112. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29632264 Free PMC article. Review.
-
Brainless but Multi-Headed: Decision Making by the Acellular Slime Mould Physarum polycephalum.J Mol Biol. 2015 Nov 20;427(23):3734-43. doi: 10.1016/j.jmb.2015.07.007. Epub 2015 Jul 17. J Mol Biol. 2015. PMID: 26189159 Review.
Cited by
-
Learning in the Single-Cell Organism Physarum polycephalum: Effect of Propofol.Int J Mol Sci. 2023 Mar 27;24(7):6287. doi: 10.3390/ijms24076287. Int J Mol Sci. 2023. PMID: 37047260 Free PMC article.
-
Dispersive transport dynamics in porous media emerge from local correlations.Nat Commun. 2022 Oct 6;13(1):5885. doi: 10.1038/s41467-022-33485-5. Nat Commun. 2022. PMID: 36202817 Free PMC article.
-
A slime mold's remembrance of things past.Proc Natl Acad Sci U S A. 2021 Apr 6;118(14):e2102056118. doi: 10.1073/pnas.2102056118. Proc Natl Acad Sci U S A. 2021. PMID: 33737448 Free PMC article. No abstract available.
-
Thoughts from the forest floor: a review of cognition in the slime mould Physarum polycephalum.Anim Cogn. 2023 Nov;26(6):1783-1797. doi: 10.1007/s10071-023-01782-1. Epub 2023 May 11. Anim Cogn. 2023. PMID: 37166523 Free PMC article. Review.
-
Hydrophobic Barriers for Directing Physarum polycephalum Propulsion and Navigation.ACS Omega. 2023 Oct 25;8(44):41649-41654. doi: 10.1021/acsomega.3c05560. eCollection 2023 Nov 7. ACS Omega. 2023. PMID: 37970039 Free PMC article.
References
-
- Nairne J. S., VanArsdall J. E., Pandeirada J. N., Blunt J. R., Adaptive memory: Enhanced location memory after survival processing. J. Exp. Psychol. Learn. Mem. Cogn. 38, 495–501 (2012). - PubMed
-
- Shohamy D., Daw N. D., Integrating memories to guide decisions. Curr Opin Behav Sci 5, 85–90 (2015).
-
- Casadesús J., D’Ari R., Memory in bacteria and phage. Bioessays 24, 512–518 (2002). - PubMed
-
- Kinoshita T., Seki M., Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol. 55, 1859–1863 (2014). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

