Test accuracy of faecal calprotectin for inflammatory bowel disease in UK primary care: a retrospective cohort study of the IMRD-UK data
- PMID: 33619196
- PMCID: PMC7903095
- DOI: 10.1136/bmjopen-2020-044177
Test accuracy of faecal calprotectin for inflammatory bowel disease in UK primary care: a retrospective cohort study of the IMRD-UK data
Abstract
Objective: To estimate the test accuracy of faecal calprotectin (FC) for inflammatory bowel disease (IBD) in the primary care setting using routine electronic health records.
Design: Retrospective cohort test accuracy study.
Setting: UK primary care.
Participants: 5970 patients (≥18 years) without a previous IBD diagnosis and with a first FC test between 1 January 2006 and 31 December 2016. We excluded multiple tests and tests without numeric results in units of µg/g.
Intervention: FC testing for the diagnosis of IBD. Disease status was confirmed by a recorded diagnostic code and/or a drug code of an IBD-specific medication at three time points after the FC test date.
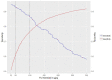
Main outcome measures: Sensitivity, specificity, and positive and negative predictive values for the differential of IBD versus non-IBD and IBD versus irritable bowel syndrome (IBS) at the 50 and 100 µg/g thresholds.
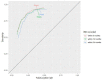
Results: 5970 patients met the inclusion criteria and had at least 6 months of follow-up data after FC testing. 1897 had an IBS diagnosis, 208 had an IBD diagnosis, 31 had a colorectal cancer diagnosis, 80 had more than one diagnosis and 3754 had no subsequent diagnosis. Sensitivity, specificity, and positive and negative predictive values were 92.9% (88.6% to 95.6%), 61.5% (60.2% to 62.7%), 8.1% (7.1% to 9.2%) and 99.6% (99.3% to 99.7%), respectively, at the threshold of 50 µg/g. Raising the threshold to 100 µg/g missed less than 7% additional IBD cases. Longer follow-up had no effect on test accuracy. Overall, uncertainty was greater for specificity than sensitivity. General practitioners' (GPs') referral decisions did not follow the anticipated clinical pathways in national guidance.
Conclusions: GPs can be confident in excluding IBD on the basis of a negative FC test in a population with low pretest risk but should interpret a positive test with caution. The applicability of national guidance to general practice needs to be improved.
Keywords: gastroenterology; inflammatory bowel disease; primary care.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare the following: KF is funded by the NIHR through a doctoral research fellowship. ST-P reports grants from the NIHR fellowship for Karoline Freemen. AC is supported by the NIHR ARC West Midlands initiative. BHW received grants from the Medical Research Council. RR has no conflicts to declare.
Figures
References
-
- Schröder O, Naumann M, Shastri Y, et al. . Prospective evaluation of faecal neutrophil-derived proteins in identifying intestinal inflammation: combination of parameters does not improve diagnostic accuracy of calprotectin. Aliment Pharmacol Ther 2007;26:1035–42. 10.1111/j.1365-2036.2007.03457.x - DOI - PubMed
-
- The Gastroenterological Society of Australia . Australian guidelines for general practitioners and physicians: inflammatory bowel disease, 2017. Available: http://cart.gesa.org.au/membes/files/Clinical%20Guidelines%20and%20Updat... [Accessed 08 Oct 2018].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
