The influence of spaceflight and simulated microgravity on bacterial motility and chemotaxis
- PMID: 33619250
- PMCID: PMC7900230
- DOI: 10.1038/s41526-021-00135-x
The influence of spaceflight and simulated microgravity on bacterial motility and chemotaxis
Abstract
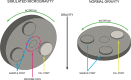
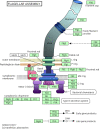
As interest in space exploration rises, there is a growing need to quantify the impact of microgravity on the growth, survival, and adaptation of microorganisms, including those responsible for astronaut illness. Motility is a key microbial behavior that plays important roles in nutrient assimilation, tissue localization and invasion, pathogenicity, biofilm formation, and ultimately survival. Very few studies have specifically looked at the effects of microgravity on the phenotypes of microbial motility. However, genomic and transcriptomic studies give a broad general picture of overall gene expression that can be used to predict motility phenotypes based upon selected genes, such as those responsible for flagellar synthesis and function and/or taxis. In this review, we focus on specific strains of Gram-negative bacteria that have been the most studied in this context. We begin with a discussion of Earth-based microgravity simulation systems and how they may affect the genes and phenotypes of interest. We then summarize results from both Earth- and space-based systems showing effects of microgravity on motility-related genes and phenotypes.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Benoit, M. R. & Klaus, D. M. Microgravity, bacteria, and the influence of motility. Adv. Space Res.39, 1225-1232 (2007).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

