Unravelling the structural complexity of glycolipids with cryogenic infrared spectroscopy
- PMID: 33619275
- PMCID: PMC7900115
- DOI: 10.1038/s41467-021-21480-1
Unravelling the structural complexity of glycolipids with cryogenic infrared spectroscopy
Abstract
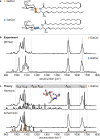
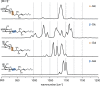
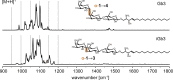
Glycolipids are complex glycoconjugates composed of a glycan headgroup and a lipid moiety. Their modular biosynthesis creates a vast amount of diverse and often isomeric structures, which fulfill highly specific biological functions. To date, no gold-standard analytical technique can provide a comprehensive structural elucidation of complex glycolipids, and insufficient tools for isomer distinction can lead to wrong assignments. Herein we use cryogenic gas-phase infrared spectroscopy to systematically investigate different kinds of isomerism in immunologically relevant glycolipids. We show that all structural features, including isomeric glycan headgroups, anomeric configurations and different lipid moieties, can be unambiguously resolved by diagnostic spectroscopic fingerprints in a narrow spectral range. The results allow for the characterization of isomeric glycolipid mixtures and biological applications.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Lipid and Glycolipid Isomer Analyses Using Ultra-High Resolution Ion Mobility Spectrometry Separations.Int J Mol Sci. 2017 Jan 18;18(1):183. doi: 10.3390/ijms18010183. Int J Mol Sci. 2017. PMID: 28106768 Free PMC article.
-
IR action spectroscopy of glycosaminoglycan oligosaccharides.Anal Bioanal Chem. 2020 Jan;412(3):533-537. doi: 10.1007/s00216-019-02327-7. Epub 2019 Dec 18. Anal Bioanal Chem. 2020. PMID: 31853603 Free PMC article.
-
Synthesis and NKT cell stimulating properties of fluorophore- and biotin-appended 6"-amino-6"-deoxy-galactosylceramides.Org Lett. 2002 Apr 18;4(8):1267-70. doi: 10.1021/ol025565+. Org Lett. 2002. PMID: 11950339
-
Infrared spectroscopy of glycolipids.Chem Phys Lipids. 1998 Nov;96(1-2):23-40. doi: 10.1016/s0009-3084(98)00078-4. Chem Phys Lipids. 1998. PMID: 9871980 Review.
-
Dry Thermotropic Glycolipid Self-Assembly:A Review.J Oleo Sci. 2018 Jun 1;67(6):651-668. doi: 10.5650/jos.ess17261. Epub 2018 May 15. J Oleo Sci. 2018. PMID: 29760332 Review.
Cited by
-
In-Depth Structural Characterization and Quantification of Cerebrosides and Glycosphingosines with Gas-Phase Ion Chemistry.Anal Chem. 2021 May 18;93(19):7332-7340. doi: 10.1021/acs.analchem.1c01021. Epub 2021 May 6. Anal Chem. 2021. PMID: 33957046 Free PMC article.
-
Identifying Mixtures of Isomeric Human Milk Oligosaccharides by the Decomposition of IR Spectral Fingerprints.Anal Chem. 2021 Nov 9;93(44):14730-14736. doi: 10.1021/acs.analchem.1c03190. Epub 2021 Oct 27. Anal Chem. 2021. PMID: 34704745 Free PMC article.
-
Unveiling Glycerolipid Fragmentation by Cryogenic Infrared Spectroscopy.J Am Chem Soc. 2021 Sep 15;143(36):14827-14834. doi: 10.1021/jacs.1c06944. Epub 2021 Sep 2. J Am Chem Soc. 2021. PMID: 34473927 Free PMC article.
-
Emerging scientists in analytical sciences: Carla Kirschbaum.Anal Sci Adv. 2022 Oct 21;3(9-10):255-257. doi: 10.1002/ansa.202200037. eCollection 2022 Oct. Anal Sci Adv. 2022. PMID: 38716267 Free PMC article. No abstract available.
-
Nanopore-based glycan sequencing: state of the art and future prospects.Chem Sci. 2024 Apr 3;15(17):6229-6243. doi: 10.1039/d4sc01466a. eCollection 2024 May 1. Chem Sci. 2024. PMID: 38699252 Free PMC article. Review.
References
-
- Schnaar, R. L. & Kinoshita, T., Essentials of Glycobiology Ch. 11 (Cold Spring Harbor Laboratory Press, 2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

