Identification of resistance pathways and therapeutic targets in relapsed multiple myeloma patients through single-cell sequencing
- PMID: 33619369
- PMCID: PMC7612793
- DOI: 10.1038/s41591-021-01232-w
Identification of resistance pathways and therapeutic targets in relapsed multiple myeloma patients through single-cell sequencing
Abstract
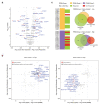
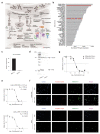
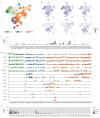
Multiple myeloma (MM) is a neoplastic plasma-cell disorder characterized by clonal proliferation of malignant plasma cells. Despite extensive research, disease heterogeneity within and between treatment-resistant patients is poorly characterized. In the present study, we conduct a prospective, multicenter, single-arm clinical trial (NCT04065789), combined with longitudinal single-cell RNA-sequencing (scRNA-seq) to study the molecular dynamics of MM resistance mechanisms. Newly diagnosed MM patients (41), who either failed to respond or experienced early relapse after a bortezomib-containing induction regimen, were enrolled to evaluate the safety and efficacy of a daratumumab, carfilzomib, lenalidomide and dexamethasone combination. The primary clinical endpoint was safety and tolerability. Secondary endpoints included overall response rate, progression-free survival and overall survival. Treatment was safe and well tolerated; deep and durable responses were achieved. In prespecified exploratory analyses, comparison of 41 primary refractory and early relapsed patients, with 11 healthy subjects and 15 newly diagnosed MM patients, revealed new MM molecular pathways of resistance, including hypoxia tolerance, protein folding and mitochondria respiration, which generalized to larger clinical cohorts (CoMMpass). We found peptidylprolyl isomerase A (PPIA), a central enzyme in the protein-folding response pathway, as a potential new target for resistant MM. CRISPR-Cas9 deletion of PPIA or inhibition of PPIA with a small molecule inhibitor (ciclosporin) significantly sensitizes MM tumor cells to proteasome inhibitors. Together, our study defines a roadmap for integrating scRNA-seq in clinical trials, identifies a signature of highly resistant MM patients and discovers PPIA as a potent therapeutic target for these tumors.
Conflict of interest statement
The clinical trial was an investigator-initiated study (IIS); as such, Y.C. acted as sponsor for the study. The IIS was approved by Amgen, who provided funding and supply of the clinical drug CFZ. Y.C. is a consultant for Janssen, Amgen, Neopharm, Takeda and Madison. A patent application has been filed related to this work. The other authors declare no competing interests.
Figures













Comment in
-
Single-cell RNA sequencing: one step closer to the clinic.Nat Med. 2021 Mar;27(3):375-376. doi: 10.1038/s41591-021-01276-y. Nat Med. 2021. PMID: 33664491 No abstract available.
References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

