Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial
- PMID: 33619370
- PMCID: PMC8052297
- DOI: 10.1038/s41591-021-01256-2
Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial
Abstract
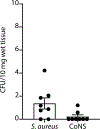
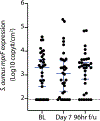
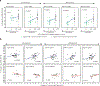
Staphylococcus aureus colonizes patients with atopic dermatitis (AD) and exacerbates disease by promoting inflammation. The present study investigated the safety and mechanisms of action of Staphylococcus hominis A9 (ShA9), a bacterium isolated from healthy human skin, as a topical therapy for AD. ShA9 killed S. aureus on the skin of mice and inhibited expression of a toxin from S. aureus (psmα) that promotes inflammation. A first-in-human, phase 1, double-blinded, randomized 1-week trial of topical ShA9 or vehicle on the forearm skin of 54 adults with S. aureus-positive AD (NCT03151148) met its primary endpoint of safety, and participants receiving ShA9 had fewer adverse events associated with AD. Eczema severity was not significantly different when evaluated in all participants treated with ShA9 but a significant decrease in S. aureus and increased ShA9 DNA were seen and met secondary endpoints. Some S. aureus strains on participants were not directly killed by ShA9, but expression of mRNA for psmα was inhibited in all strains. Improvement in local eczema severity was suggested by post-hoc analysis of participants with S. aureus directly killed by ShA9. These observations demonstrate the safety and potential benefits of bacteriotherapy for AD.
Conflict of interest statement
Competing interests
T.N. and R.L.G. are co-inventors of UCSD technology related to the bacterial antimicrobial peptides discussed herein. R.L.G. is co-founder and has equity interest in MatriSys Bioscience and Sente Inc. A.K.R.S.’s co-authorship of this publication does not necessarily constitute endorsement by the NIAID, the NIH or any other agency of the US government. All other authors declare no conflicts of interest.
Figures



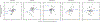
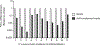
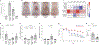



References
-
- Kapoor R et al. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J. Am. Acad. Dermatol 58, 68–73 (2008). - PubMed
-
- Bieber T Atopic dermatitis. N. Engl. J. Med 358, 1483–1494 (2008). - PubMed
-
- Nutten S Atopic dermatitis: global epidemiology and risk factors. Ann. Nutr. Metab 66, 8–16 (2015). - PubMed
-
- Reed B & Blaiss MS The burden of atopic dermatitis. Allergy Asthma Proc 39, 406–410 (2018). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

