Direct cell reprogramming: approaches, mechanisms and progress
- PMID: 33619373
- PMCID: PMC8161510
- DOI: 10.1038/s41580-021-00335-z
Direct cell reprogramming: approaches, mechanisms and progress
Abstract
The reprogramming of somatic cells with defined factors, which converts cells from one lineage into cells of another, has greatly reshaped our traditional views on cell identity and cell fate determination. Direct reprogramming (also known as transdifferentiation) refers to cell fate conversion without transitioning through an intermediary pluripotent state. Given that the number of cell types that can be generated by direct reprogramming is rapidly increasing, it has become a promising strategy to produce functional cells for therapeutic purposes. This Review discusses the evolution of direct reprogramming from a transcription factor-based method to a small-molecule-driven approach, the recent progress in enhancing reprogrammed cell maturation, and the challenges associated with in vivo direct reprogramming for translational applications. It also describes our current understanding of the molecular mechanisms underlying direct reprogramming, including the role of transcription factors, epigenetic modifications, non-coding RNAs, and the function of metabolic reprogramming, and highlights novel insights gained from single-cell omics studies.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
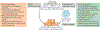
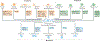


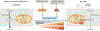

References
-
- Waddington CH The Strategy of the Genes. A Discussion of Some Aspects of Theoretical Biology. With an Appendix by H. Kacser (George Allen & Unwin, Ltd., 1957).
-
-
Davis RL, Weintraub H & Lassar AB Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51, 987–1000 (1987).
Davis et al. demonstrated, for the first time, that the overexpression of one transcription factor could rewrite cell fate in vitro.
-
-
- Takahashi K & Yamanaka S Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006). - PubMed
-
- Yamanaka S Induced pluripotent stem cells: past, present, and future. Cell Stem Cell 10, 678–684 (2012). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

