Long-term maturation of human cortical organoids matches key early postnatal transitions
- PMID: 33619405
- PMCID: PMC8109149
- DOI: 10.1038/s41593-021-00802-y
Long-term maturation of human cortical organoids matches key early postnatal transitions
Abstract
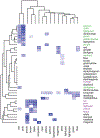
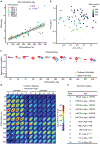
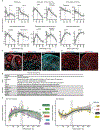
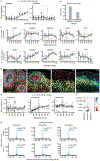
Human stem-cell-derived models provide the promise of accelerating our understanding of brain disorders, but not knowing whether they possess the ability to mature beyond mid- to late-fetal stages potentially limits their utility. We leveraged a directed differentiation protocol to comprehensively assess maturation in vitro. Based on genome-wide analysis of the epigenetic clock and transcriptomics, as well as RNA editing, we observe that three-dimensional human cortical organoids reach postnatal stages between 250 and 300 days, a timeline paralleling in vivo development. We demonstrate the presence of several known developmental milestones, including switches in the histone deacetylase complex and NMDA receptor subunits, which we confirm at the protein and physiological levels. These results suggest that important components of an intrinsic in vivo developmental program persist in vitro. We further map neurodevelopmental and neurodegenerative disease risk genes onto in vitro gene expression trajectories to provide a resource and webtool (Gene Expression in Cortical Organoids, GECO) to guide disease modeling.
Conflict of interest statement
Competing Interests Statement
S.P.P. is listed on a patent held by Stanford University that covers the generation of region-specific brain organoids (U.S. patent 62/477,858). All other authors declare no competing interests.
Figures









References
-
- Pasca SP The rise of three-dimensional human brain cultures. Nature 553, 437–445 (2018). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

