This is a preprint.
In depth analysis of patients with severe SARS-CoV-2 in sub-Saharan Africa demonstrates distinct clinical and immunological profiles
- PMID: 33619502
- PMCID: PMC7899472
- DOI: 10.1101/2021.02.15.21251753
In depth analysis of patients with severe SARS-CoV-2 in sub-Saharan Africa demonstrates distinct clinical and immunological profiles
Update in
-
Distinct clinical and immunological profiles of patients with evidence of SARS-CoV-2 infection in sub-Saharan Africa.Nat Commun. 2021 Jun 11;12(1):3554. doi: 10.1038/s41467-021-23267-w. Nat Commun. 2021. PMID: 34117221 Free PMC article.
Abstract
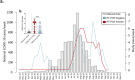
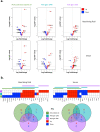
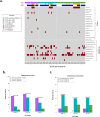
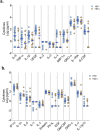
Although the COVID-19 pandemic has left no country untouched there has been limited research to understand clinical and immunological responses in African populations. Here we comprehensively characterise patients hospitalised with suspected or confirmed COVID-19, and healthy community controls. PCR-confirmed COVID-19 participants were more likely to receive dexamethasone and a beta-lactam antibiotic, and survive to hospital discharge than PCR-/IgG+ and PCR-/IgG-participants. PCR-/IgG+ participants exhibited a nasal and systemic cytokine signature analogous to PCR-confirmed COVID-19 participants, but increased propensity for Staphylococcus aureus and Streptococcus pneumoniae colonisation. We did not find evidence that HIV co-infection in COVID-19 participants was associated with mortality or altered cytokine responses. The nasal immune signature in PCR-/IgG+ and PCR-confirmed COVID-19 participants was distinct and predominated by chemokines and neutrophils. In addition, PCR-/IgG+ individuals with high COVID-19 clinical suspicion had inflammatory profiles analogous to PCR-confirmed disease and potentially represent a target population for COVID-19 treatment strategies.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures


References
-
- Government SA. Update on Covid-19 2020. [Available from: https://sacoronavirus.co.za.
-
- Kuehn BM. Africa Succeeded Against COVID-19’s First Wave, but the Second Wave Brings New Challenges. Jama. 2021;325(4):327–8. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
