RADTHYR: an open-label, single-arm, prospective multicenter phase II trial of Radium-223 for the treatment of bone metastases from radioactive iodine refractory differentiated thyroid cancer
- PMID: 33619600
- PMCID: PMC8426251
- DOI: 10.1007/s00259-021-05229-y
RADTHYR: an open-label, single-arm, prospective multicenter phase II trial of Radium-223 for the treatment of bone metastases from radioactive iodine refractory differentiated thyroid cancer
Abstract
Purpose: This is the first prospective trial evaluating the efficacy of alpha emitter Radium-223 in patients with bone metastases from radioactive iodine (RAI) refractory (RAIR) differentiated thyroid cancer.
Methods: RADTHYR is a multicenter, single-arm prospective Simon two-stage phase II trial (NCT02390934). The primary objective was to establish the efficacy of three administrations of 55 kBq/kg of Radium-223 by 18F-FDG PET/CT according to PERCIST criteria. Secondary objectives were to establish the efficacy of six administrations of Radium-223 by 18F-FDG PET/CT, 99mTc-HMDP bone scan and 18FNa PET/CT, clinical benefits, changes in serum bone markers, thyroglobulin levels, and safety.
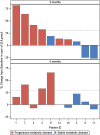
Results: Ten patients were enrolled between July 2015 and December 2017 (4 M; median age 74 years). Prior to Radium-223 administration, patients received a median RAI cumulative activity of 15 GBq (7.4-35.6), external radiation therapy (n = 9), bone surgery (n = 8), cimentoplasty (n = 5), and cryoablation (n = 2). 18F-FDG PET/CT showed stable disease (SD) in 4/10 and progressive disease (PD) in 6/10 cases after three administrations and SD in 4/10, PD in 5/10 cases, and 1/10 non-evaluable (NE) case after six administrations. After six injections, 99mTc-HMDP bone scan showed SD in 9 cases and was NE in 1 case; 18FNa PET/CT showed SD in 8 cases, partial response (PR) in 1 case, and was NE in 1 case. No significant clinical benefits were reported during the study. A skeletal event occurred in 6 patients (median time without skeletal event of 12.1 months). Seventy-seven adverse events were reported during treatment (7 of grade 3-4). Three patients developed an acute myeloid, a promyelocytic, and a chronic myeloid leukemia after the last Radium-223 administration considered as drug-related.
Conclusion: The trial was stopped after interim analysis for lack of response of bone metastases from RAIR thyroid cancer to Radium-223. Severe hematological toxicity was observed in patients heavily pretreated with RAI and external radiation.
Trial registration number: NCT02390934. Registration date 18.03.2015.
Keywords: Alpha emitters; Bone metastases; Leukemia; Radium-223; Refractory thyroid cancer.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
68Ga-DOTA-RGD2 Positron Emission Tomography/Computed Tomography in Radioiodine Refractory Thyroid Cancer: Prospective Comparison of Diagnostic Accuracy with 18F-FDG Positron Emission Tomography/Computed Tomography and Evaluation Toward Potential Theranostics.Thyroid. 2020 Apr;30(4):557-567. doi: 10.1089/thy.2019.0450. Epub 2020 Jan 23. Thyroid. 2020. PMID: 31870227
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Comparison of the diagnostic and prognostic values of 99mTc-MDP-planar bone scintigraphy, 131I-SPECT/CT and 18F-FDG-PET/CT for the detection of bone metastases from differentiated thyroid cancer.Nucl Med Commun. 2012 Dec;33(12):1232-42. doi: 10.1097/MNM.0b013e328358d9c0. Nucl Med Commun. 2012. PMID: 23111353
-
Evaluating radium-223 response in metastatic castration-resistant prostate cancer with imaging.Asia Pac J Clin Oncol. 2018 Nov;14 Suppl 5:16-23. doi: 10.1111/ajco.13058. Asia Pac J Clin Oncol. 2018. PMID: 30489033 Review.
-
Respective roles of thyroglobulin, radioiodine imaging, and positron emission tomography in the assessment of thyroid cancer.Semin Nucl Med. 2006 Jul;36(3):194-205. doi: 10.1053/j.semnuclmed.2006.03.002. Semin Nucl Med. 2006. PMID: 16762610 Review.
Cited by
-
Recurrent Extraneural Metastatic Medulloblastoma in an Adult Presenting With a Superscan and Treated With Radium-223.Cureus. 2023 Feb 7;15(2):e34732. doi: 10.7759/cureus.34732. eCollection 2023 Feb. Cureus. 2023. PMID: 36909024 Free PMC article.
-
Advances in Functional Imaging of Differentiated Thyroid Cancer.Cancers (Basel). 2021 Sep 23;13(19):4748. doi: 10.3390/cancers13194748. Cancers (Basel). 2021. PMID: 34638232 Free PMC article. Review.
-
Alpha-Emitting Radionuclides: Current Status and Future Perspectives.Pharmaceuticals (Basel). 2024 Jan 8;17(1):76. doi: 10.3390/ph17010076. Pharmaceuticals (Basel). 2024. PMID: 38256909 Free PMC article. Review.
References
-
- National Cancer Institute: Surveillance, Epidemiology and ERP. Cancer Stat Facts: Thyroid Cancer [Internet]. 2015 [cited 2019 Aug 16]. Available at: https://seer.cancer.gov/statfacts/html/thyro.html
-
- Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

