Botulinum Toxin Versus Placebo: A Meta-Analysis of Treatment and Quality-of-life Outcomes for Hyperhidrosis
- PMID: 33619611
- PMCID: PMC8316174
- DOI: 10.1007/s00266-021-02140-7
Botulinum Toxin Versus Placebo: A Meta-Analysis of Treatment and Quality-of-life Outcomes for Hyperhidrosis
Abstract
Aims: This study aims at assessing the treatment effect, disease severity and quality-of-life outcomes of botulinum toxin (BTX) injections for focal hyperhidrosis.
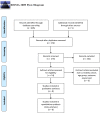
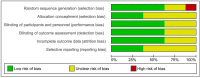
Methods: We included randomized controlled trials of BTX injections compared with placebo for patients with primary or secondary focal hyperhidrosis. PubMed, Embase and the Cochrane Library were searched to August 2020. Gravimetric sweat rate reduction, disease severity measured by Hyperhidrosis Disease Severity Scale and quality-of-life assessment measured by Dermatology Life Quality Index were the outcomes of interest. Cochrane risk-of-bias tools were employed for quality assessment of given randomized controlled trials.
Results: Eight studies met our inclusion criteria (n=937). Overall, risk bias was mixed and mostly moderate. BTX injections showed reduced risk in comparison with placebo for the gravimetric quantitative sweat reduction of > 50 % from baseline (risk difference: 0.63, 95% CI 0.51 to 0.74). Additionally, improvements were seen for disease severity and quality-of-life assessments evaluated by Hyperhidrosis Disease Severity Score reduction of ≥ 2 points (risk difference: 0.56, 95% CI 0.42 to 0.69) and mean change in Dermatology Life Quality Index (mean difference: - 5.55, 95% CI - 7.11 to - 3.98). The acquired data were insufficient to assess for long-term outcomes and limited to an eight-week follow-up period.
Conclusions: In focal axillary hyperhidrosis, BTX significantly reduces sweat production and yields superior outcomes in assessments of disease severity and quality-of-life. However, the quality-of-evidence is overall moderate and included studies account for short-term trial periods only. Further studies assessing BTX in comparison with first-line treatments for hyperhidrosis are warranted.
Level of evidence iii: This journal requires that authors assign a level of evidence to each article. For a full description of these evidence-based medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266 .
Keywords: Botox; Botulinum toxin; Hyperhidrosis; Meta-analysis; Randomized controlled trial.
© 2021. The Author(s).
Conflict of interest statement
The authors declare they have no conflicts of interest to disclose.
Figures


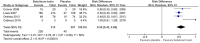

Similar articles
-
Efficacy and Safety of Botulinum Toxin Type A in Primary Axillary Hyperhidrosis: A Meta-analysis and Systematic Review.Aesthetic Plast Surg. 2025 Jun 11. doi: 10.1007/s00266-025-04909-6. Online ahead of print. Aesthetic Plast Surg. 2025. PMID: 40500510 Review.
-
Botulinum toxin type a is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study.Arch Dermatol. 2003 Jun;139(6):731-6. doi: 10.1001/archderm.139.6.731. Arch Dermatol. 2003. PMID: 12810503 Clinical Trial.
-
Effect of botulinum toxin type A on quality of life measures in patients with excessive axillary sweating: a randomized controlled trial.Br J Dermatol. 2002 Dec;147(6):1218-26. doi: 10.1046/j.1365-2133.2002.05059.x. Br J Dermatol. 2002. PMID: 12452874 Clinical Trial.
-
Long-Term Safety and Efficacy of Botulinum Toxin A Treatment in Adolescent Patients with Axillary Bromhidrosis.Aesthetic Plast Surg. 2018 Apr;42(2):560-564. doi: 10.1007/s00266-018-1075-4. Epub 2018 Jan 17. Aesthetic Plast Surg. 2018. PMID: 29344685
-
Interventions for hyperhidrosis in secondary care: a systematic review and value-of-information analysis.Health Technol Assess. 2017 Dec;21(80):1-280. doi: 10.3310/hta21800. Health Technol Assess. 2017. PMID: 29271741 Free PMC article.
Cited by
-
Longitudinal Assessment of Facial Hyperhidrosis Management: Evaluating the Utility and Quality of Life Improvements following Botulinum Toxin Injection.Toxins (Basel). 2024 Jan 21;16(1):59. doi: 10.3390/toxins16010059. Toxins (Basel). 2024. PMID: 38276535 Free PMC article.
-
Treatment of Hyperhidrosis: An Update.Am J Clin Dermatol. 2022 Sep;23(5):635-646. doi: 10.1007/s40257-022-00707-x. Epub 2022 Jul 1. Am J Clin Dermatol. 2022. PMID: 35773437 Review.
-
Assessing Botulinum Toxin Effectiveness and Quality of Life in Axillary Hyperhidrosis: A One-Year Prospective Study.Diseases. 2024 Jan 3;12(1):15. doi: 10.3390/diseases12010015. Diseases. 2024. PMID: 38248366 Free PMC article.
-
Microneedling Delivery of Botulinum Toxin Versus Intradermal Injection in the Treatment of Facial Hyperhidrosis.J Clin Aesthet Dermatol. 2022 Sep;15(9):40-44. J Clin Aesthet Dermatol. 2022. PMID: 36213604 Free PMC article.
-
Molecular and Physiological Functions of PACAP in Sweat Secretion.Int J Mol Sci. 2023 Feb 26;24(5):4572. doi: 10.3390/ijms24054572. Int J Mol Sci. 2023. PMID: 36902003 Free PMC article.
References
-
- Menzinger S, Quenan S. Evaluation and management of hyperhidrosis. Rev Med Suisse. 2017;13(556):710–714. - PubMed
-
- Solish N, Bertucci V, Dansereau A, et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian hyperhidrosis advisory committee. Dermatol Surg. 2007;33(8):908–923. - PubMed
-
- Brackenrich J, Fagg C. Hyperhidrosis. In Statpearls. Treasure Island (FL): StatPearls Publishing; 2020. - PubMed
-
- Flanagan KH, King R, Glaser DA. Botulinum toxin type a versus topical 20% aluminum chloride for the treatment of moderate to severe primary focal axillary hyperhidrosis. J Drugs Dermatol. 2008;7(3):221–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

