Function of Drosophila Synaptotagmins in membrane trafficking at synapses
- PMID: 33619613
- PMCID: PMC8164606
- DOI: 10.1007/s00018-021-03788-9
Function of Drosophila Synaptotagmins in membrane trafficking at synapses
Abstract
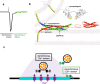
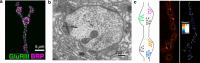
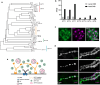
The Synaptotagmin (SYT) family of proteins play key roles in regulating membrane trafficking at neuronal synapses. Using both Ca2+-dependent and Ca2+-independent interactions, several SYT isoforms participate in synchronous and asynchronous fusion of synaptic vesicles (SVs) while preventing spontaneous release that occurs in the absence of stimulation. Changes in the function or abundance of the SYT1 and SYT7 isoforms alter the number and route by which SVs fuse at nerve terminals. Several SYT family members also regulate trafficking of other subcellular organelles at synapses, including dense core vesicles (DCV), exosomes, and postsynaptic vesicles. Although SYTs are linked to trafficking of multiple classes of synaptic membrane compartments, how and when they interact with lipids, the SNARE machinery and other release effectors are still being elucidated. Given mutations in the SYT family cause disorders in both the central and peripheral nervous system in humans, ongoing efforts are defining how these proteins regulate vesicle trafficking within distinct neuronal compartments. Here, we review the Drosophila SYT family and examine their role in synaptic communication. Studies in this invertebrate model have revealed key similarities and several differences with the predicted activity of their mammalian counterparts. In addition, we highlight the remaining areas of uncertainty in the field and describe outstanding questions on how the SYT family regulates membrane trafficking at nerve terminals.
Keywords: Drosophila; Exocytosis; Neurotransmitter release; Synapse; Synaptic vesicle; Synaptotagmin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
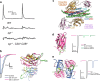

Similar articles
-
Complexin controls spontaneous and evoked neurotransmitter release by regulating the timing and properties of synaptotagmin activity.J Neurosci. 2012 Dec 12;32(50):18234-45. doi: 10.1523/JNEUROSCI.3212-12.2012. J Neurosci. 2012. PMID: 23238737 Free PMC article.
-
Drosophila Synaptotagmin 7 negatively regulates synaptic vesicle release and replenishment in a dosage-dependent manner.Elife. 2020 Apr 28;9:e55443. doi: 10.7554/eLife.55443. Elife. 2020. PMID: 32343229 Free PMC article.
-
Characterization of the role of the Synaptotagmin family as calcium sensors in facilitation and asynchronous neurotransmitter release.Proc Natl Acad Sci U S A. 2007 Aug 28;104(35):14122-7. doi: 10.1073/pnas.0706711104. Epub 2007 Aug 20. Proc Natl Acad Sci U S A. 2007. PMID: 17709738 Free PMC article.
-
The synaptotagmins: calcium sensors for vesicular trafficking.Neuroscientist. 2004 Dec;10(6):566-74. doi: 10.1177/1073858404268770. Neuroscientist. 2004. PMID: 15534041 Review.
-
Exocytosis and synaptic vesicle function.Compr Physiol. 2014 Jan;4(1):149-75. doi: 10.1002/cphy.c130021. Compr Physiol. 2014. PMID: 24692137 Review.
Cited by
-
SNARE Regulatory Proteins in Synaptic Vesicle Fusion and Recycling.Front Mol Neurosci. 2021 Aug 6;14:733138. doi: 10.3389/fnmol.2021.733138. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34421538 Free PMC article. Review.
-
Conserved expression of the zebrafish syt4 gene in GABAergic neurons in the cerebellum of adult fishes revealed by mammalian SYT4 immunoreactive-like signals.Heliyon. 2024 May 3;10(9):e30575. doi: 10.1016/j.heliyon.2024.e30575. eCollection 2024 May 15. Heliyon. 2024. PMID: 38765140 Free PMC article.
-
Genetic regulation of central synapse formation and organization in Drosophila melanogaster.Genetics. 2022 Jul 4;221(3):iyac078. doi: 10.1093/genetics/iyac078. Genetics. 2022. PMID: 35652253 Free PMC article. Review.
-
Stochastic RNA editing of the Complexin C-terminus within single neurons regulates neurotransmitter release.bioRxiv [Preprint]. 2023 May 30:2023.05.30.542887. doi: 10.1101/2023.05.30.542887. bioRxiv. 2023. Update in: Cell Rep. 2023 Sep 26;42(9):113152. doi: 10.1016/j.celrep.2023.113152. PMID: 37398117 Free PMC article. Updated. Preprint.
-
Intact Drosophila central nervous system cellular quantitation reveals sexual dimorphism.Elife. 2022 Jul 8;11:e74968. doi: 10.7554/eLife.74968. Elife. 2022. PMID: 35801638 Free PMC article.
References
-
- Katz BS. The Release of Neural Transmitter Substances (Sherrington Lecture): Bernard S. Katz: 9780853230601: Amazon.com: Books. Liverpool: Liverpool University Press; 1969.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

