Protein products of nonstop mRNA disrupt nucleolar homeostasis
- PMID: 33619693
- PMCID: PMC8065075
- DOI: 10.1007/s12192-021-01200-w
Protein products of nonstop mRNA disrupt nucleolar homeostasis
Abstract
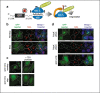
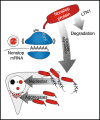
Stalled mRNA translation results in the production of incompletely synthesized proteins that are targeted for degradation by ribosome-associated quality control (RQC). Here we investigated the fate of defective proteins translated from stall-inducing, nonstop mRNA that escape ubiquitylation by the RQC protein LTN1. We found that nonstop protein products accumulated in nucleoli and this localization was driven by polylysine tracts produced by translation of the poly(A) tails of nonstop mRNA. Nucleolar sequestration increased the solubility of invading proteins but disrupted nucleoli, altering their dynamics, morphology, and resistance to stress in cell culture and intact flies. Our work elucidates how stalled translation may affect distal cellular processes and may inform studies on the pathology of diseases caused by failures in RQC and characterized by nucleolar stress.
Keywords: LTN1; Nonstop mRNA; Nucleolus; Phase separation; Protein quality control; Ribosome-associated quality control (RQC).
Figures


Similar articles
-
Ribosome-associated quality-control mechanisms from bacteria to humans.Mol Cell. 2022 Apr 21;82(8):1451-1466. doi: 10.1016/j.molcel.2022.03.038. Mol Cell. 2022. PMID: 35452614 Free PMC article. Review.
-
Rqc1 and Ltn1 Prevent C-terminal Alanine-Threonine Tail (CAT-tail)-induced Protein Aggregation by Efficient Recruitment of Cdc48 on Stalled 60S Subunits.J Biol Chem. 2016 Jun 3;291(23):12245-53. doi: 10.1074/jbc.M116.722264. Epub 2016 Apr 18. J Biol Chem. 2016. PMID: 27129255 Free PMC article.
-
Protein quality control systems associated with no-go and nonstop mRNA surveillance in yeast.Genes Cells. 2014 Jan;19(1):1-12. doi: 10.1111/gtc.12106. Epub 2013 Nov 21. Genes Cells. 2014. PMID: 24261871
-
Rkr1/Ltn1 Ubiquitin Ligase-mediated Degradation of Translationally Stalled Endoplasmic Reticulum Proteins.J Biol Chem. 2015 Jul 24;290(30):18454-66. doi: 10.1074/jbc.M115.663559. Epub 2015 Jun 8. J Biol Chem. 2015. PMID: 26055716 Free PMC article.
-
Argonaute-dependent ribosome-associated protein quality control.Trends Cell Biol. 2023 Mar;33(3):260-272. doi: 10.1016/j.tcb.2022.07.007. Epub 2022 Aug 16. Trends Cell Biol. 2023. PMID: 35981909 Review.
Cited by
-
Quality control of cytoplasmic proteins inside the nucleus.Comput Struct Biotechnol J. 2022 Aug 23;20:4618-4625. doi: 10.1016/j.csbj.2022.08.033. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36090811 Free PMC article. Review.
-
Ribosome-associated quality-control mechanisms from bacteria to humans.Mol Cell. 2022 Apr 21;82(8):1451-1466. doi: 10.1016/j.molcel.2022.03.038. Mol Cell. 2022. PMID: 35452614 Free PMC article. Review.
-
NEMF mutations in mice illustrate how Importin-β specific nuclear transport defects recapitulate neurodegenerative disease hallmarks.PLoS Genet. 2024 Sep 23;20(9):e1011411. doi: 10.1371/journal.pgen.1011411. eCollection 2024 Sep. PLoS Genet. 2024. PMID: 39312574 Free PMC article.
-
Detection and Degradation of Stalled Nascent Chains via Ribosome-Associated Quality Control.Annu Rev Biochem. 2020 Jun 20;89:417-442. doi: 10.1146/annurev-biochem-013118-110729. Annu Rev Biochem. 2020. PMID: 32569528 Free PMC article. Review.
-
Preserve or destroy: Orphan protein proteostasis and the heat shock response.J Cell Biol. 2024 Dec 2;223(12):e202407123. doi: 10.1083/jcb.202407123. Epub 2024 Nov 15. J Cell Biol. 2024. PMID: 39545954 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

