Androgen receptors in areas of the spinal cord and brainstem: A study in adult male cats
- PMID: 33619726
- PMCID: PMC8197961
- DOI: 10.1111/joa.13407
Androgen receptors in areas of the spinal cord and brainstem: A study in adult male cats
Abstract
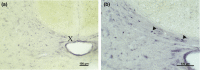
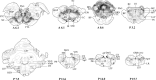
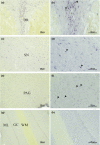
Sex hormones, including androgens and estrogens, play an important role in autonomic, reproductive and sexual behavior. The areas that are important in these behaviors lie within the spinal cord and brainstem. Relevant dysfunctional behavior in patients with altered androgen availability or androgen receptor sensitivity might be explained by the distribution of androgens and their receptors in the central nervous system. We hypothesize that autonomic dysfunction is correlated with the androgen sensitivity of spinal cord and brainstem areas responsible for autonomic functions. In this study, androgen receptor immunoreactive (AR-IR) nuclei in the spinal cord and brainstem were studied using the androgen receptor antibody PG21 in four uncastrated young adult male cats. A dense distribution of AR-IR nuclei was detected in the superior layers of the dorsal horn, including lamina I. Intensely stained nuclei, but less densely distributed, were found in lamina X and preganglionic sympathetic and parasympathetic cells of the intermediolateral cell column. Areas in the caudal brainstem showing a high density of AR-IR nuclei included the area postrema, the dorsal motor vagus nucleus and the retrotrapezoid nucleus. More cranially, the central linear nucleus in the pons contained a dense distribution of AR-IR nuclei. The mesencephalic periaqueductal gray (PAG) showed a dense distribution of AR-IR nuclei apart from the most central part of the PAG directly adjacent to the ependymal lining. Other areas in the mesencephalon with a dense distribution of AR-IR nuclei were the dorsal raphe nucleus, the retrorubral nucleus, the substantia nigra and the ventral tegmental area of Tsai. It is concluded that AR-IR nuclei are located in specific areas of the central nervous system that are involved in the control of sensory function and autonomic behavior. Furthermore, damage of these AR-IR areas might explain related dysfunction in humans.
Keywords: PG21; androgen; autonomic function; medulla oblongata; mesencephalon; spinal cord.
© 2021 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
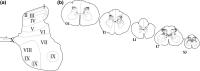
References
-
- Avendaño, C. & Verdu, A. (1992) Area 3a in the cat. I. A reevaluation of its location and architecture on the basis of Nissl, myelin, acetylcholinesterase, and cytochrome oxidase staining. Journal of Comparative Neurology, 321, 357–372. - PubMed
-
- Basaria, S. , Lieb, J. , Tang, A.M. , DeWeese, T. , Carducci, M. , Eisenberger, M. , et al. (2002) Long‐term effects of androgen deprivation therapy in prostate cancer patients. Clinical Endocrinology, 56, 779–786. - PubMed
-
- Berman, A.L. (1968) The brain stem of the cat. A cytoarchitectonic atlas with stereotaxic coodinates, 1–175.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

